B. Oocyte maturation stage
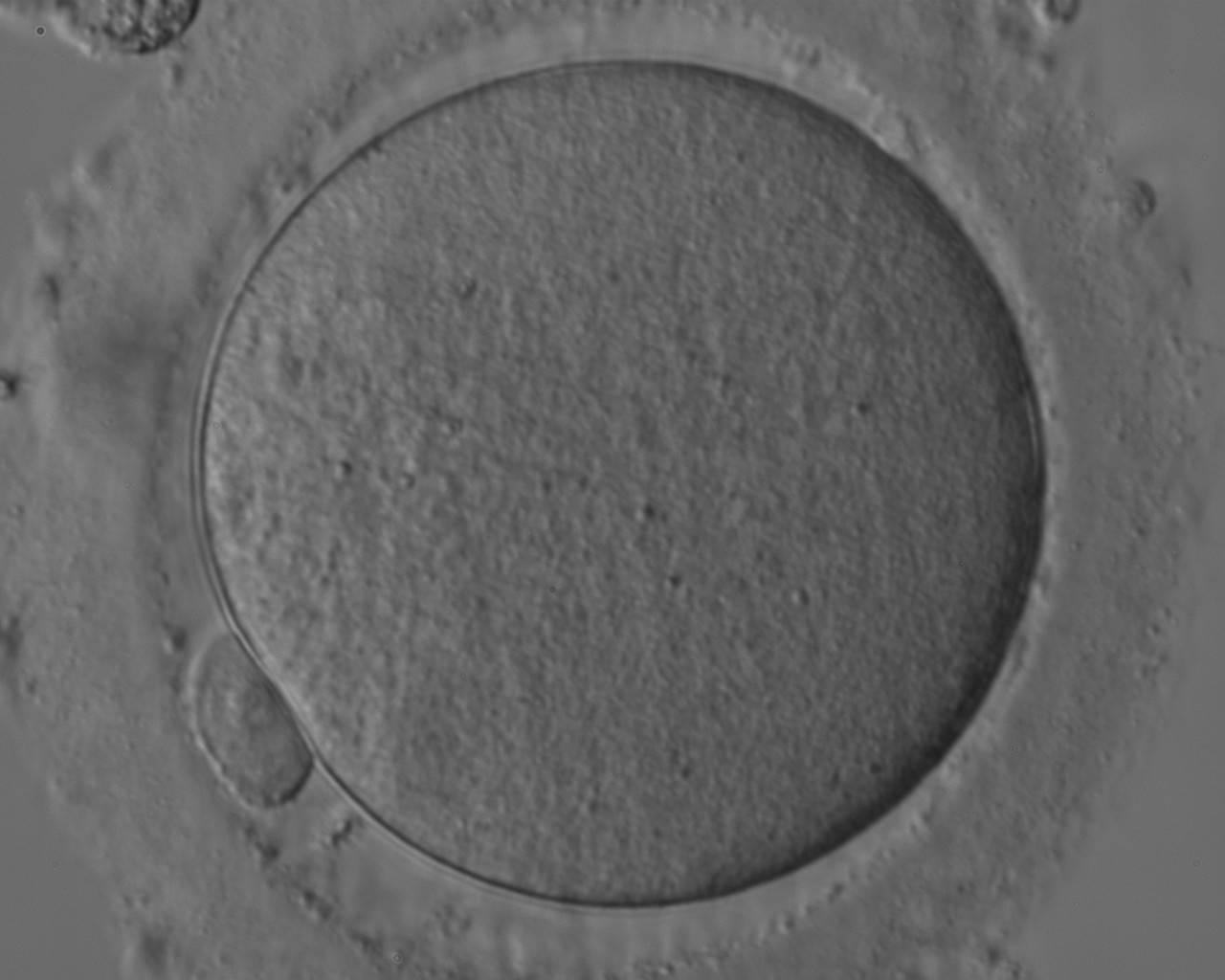
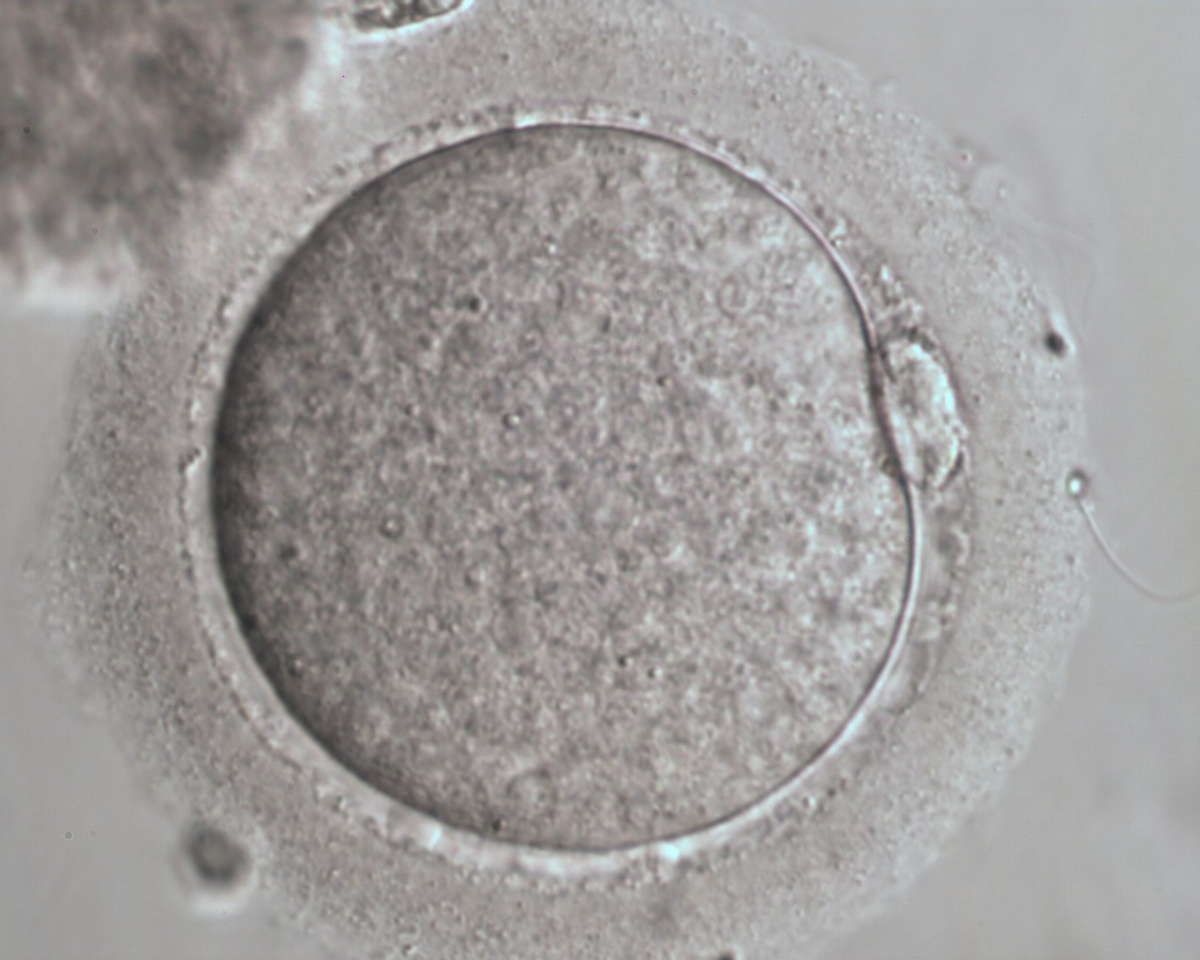
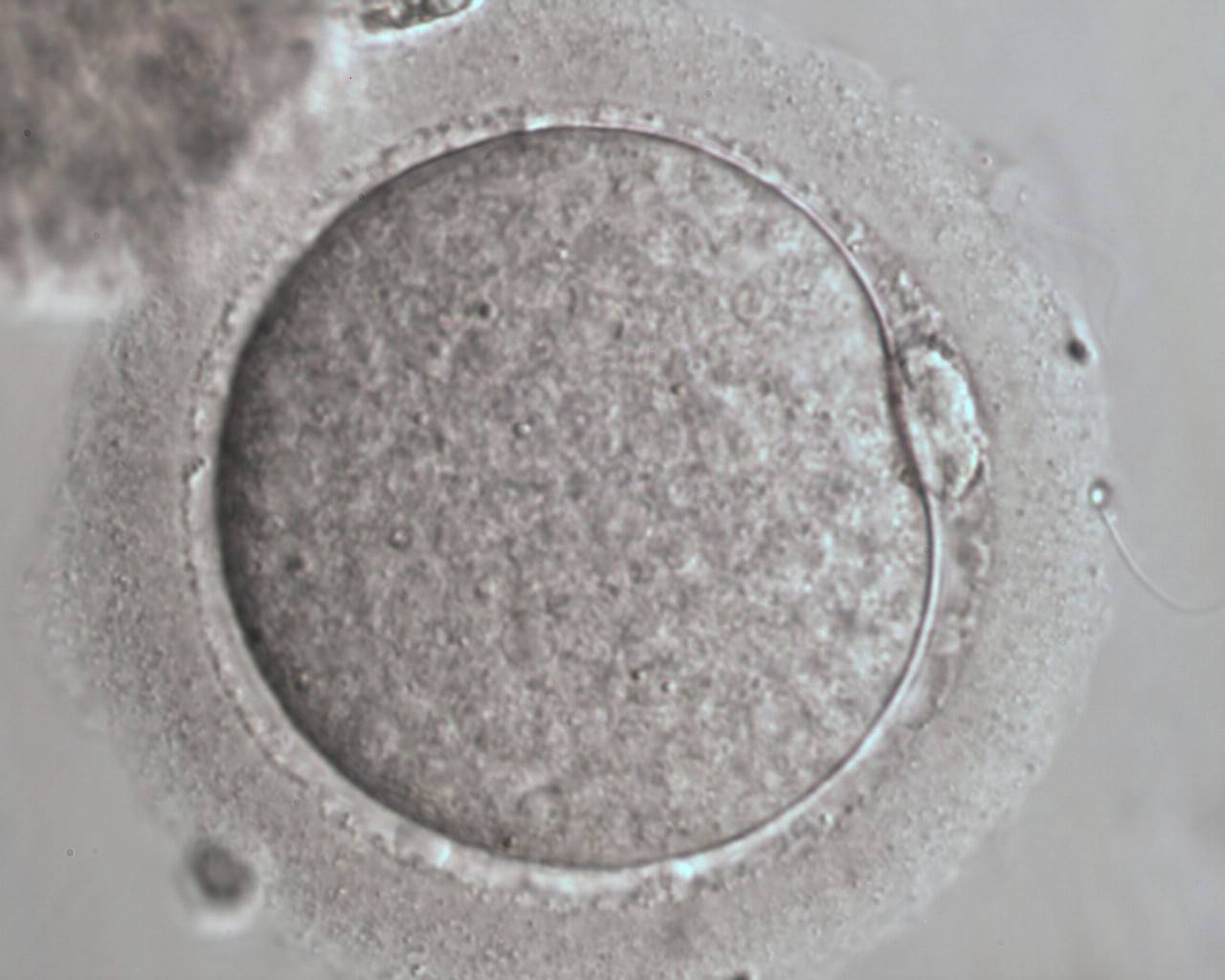
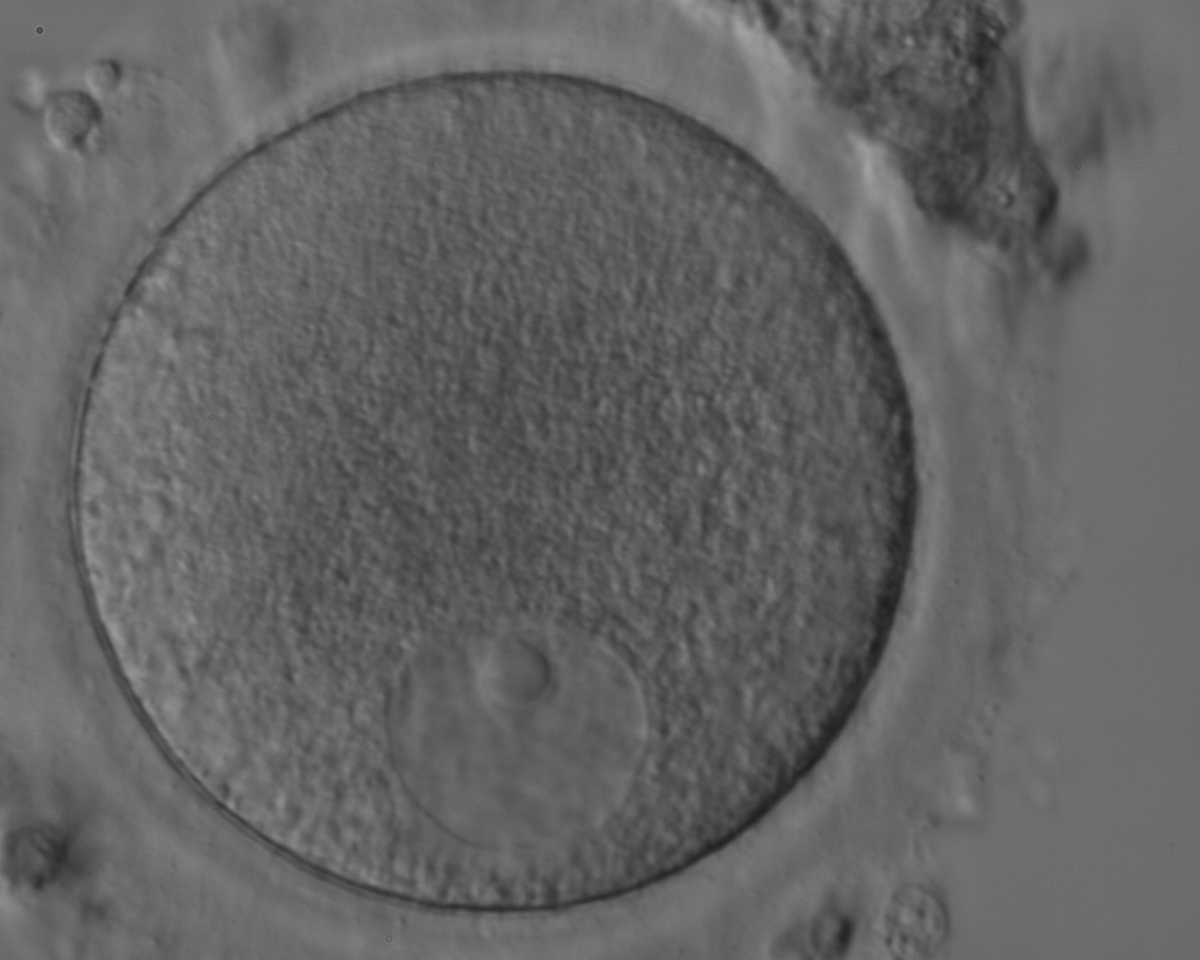
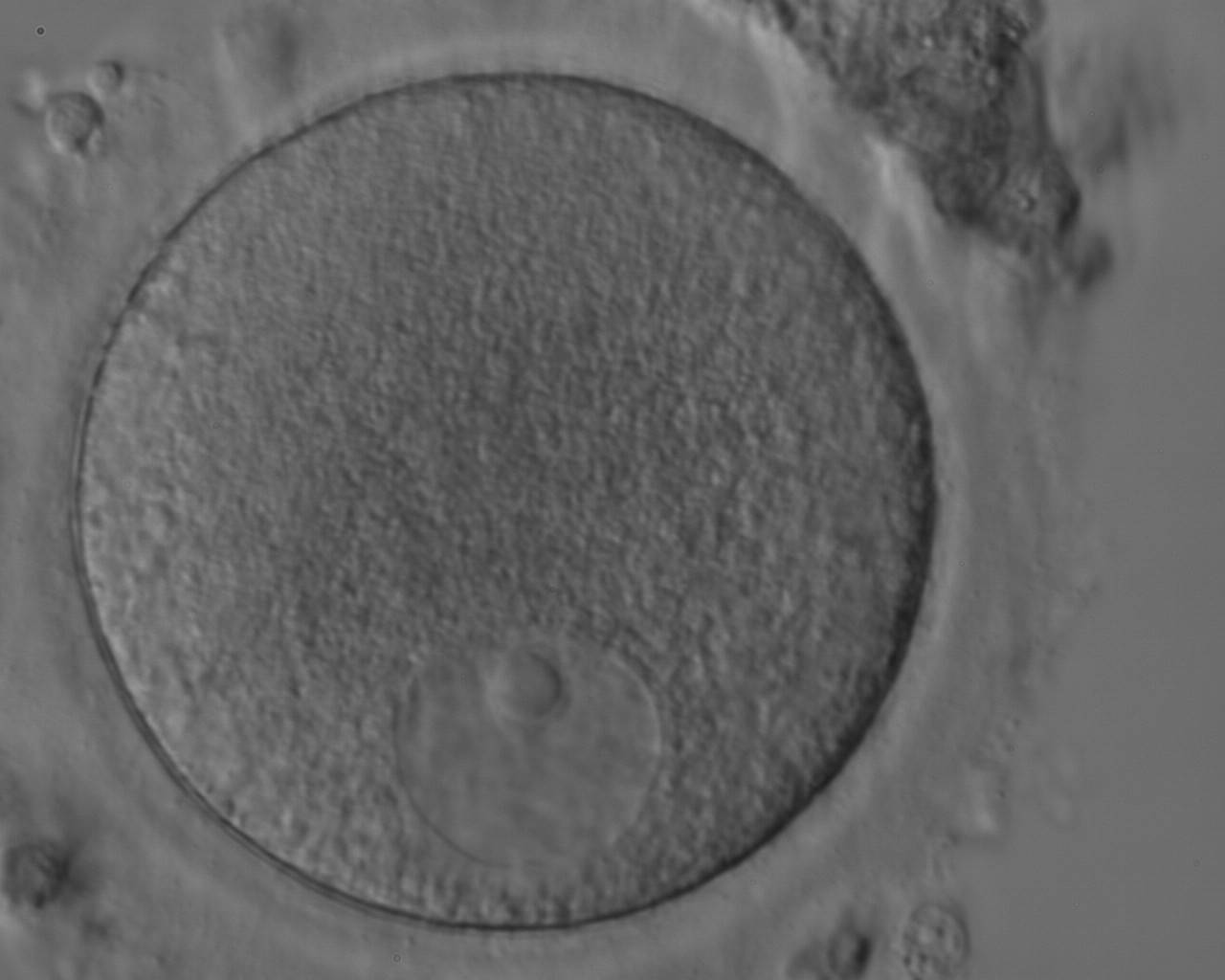
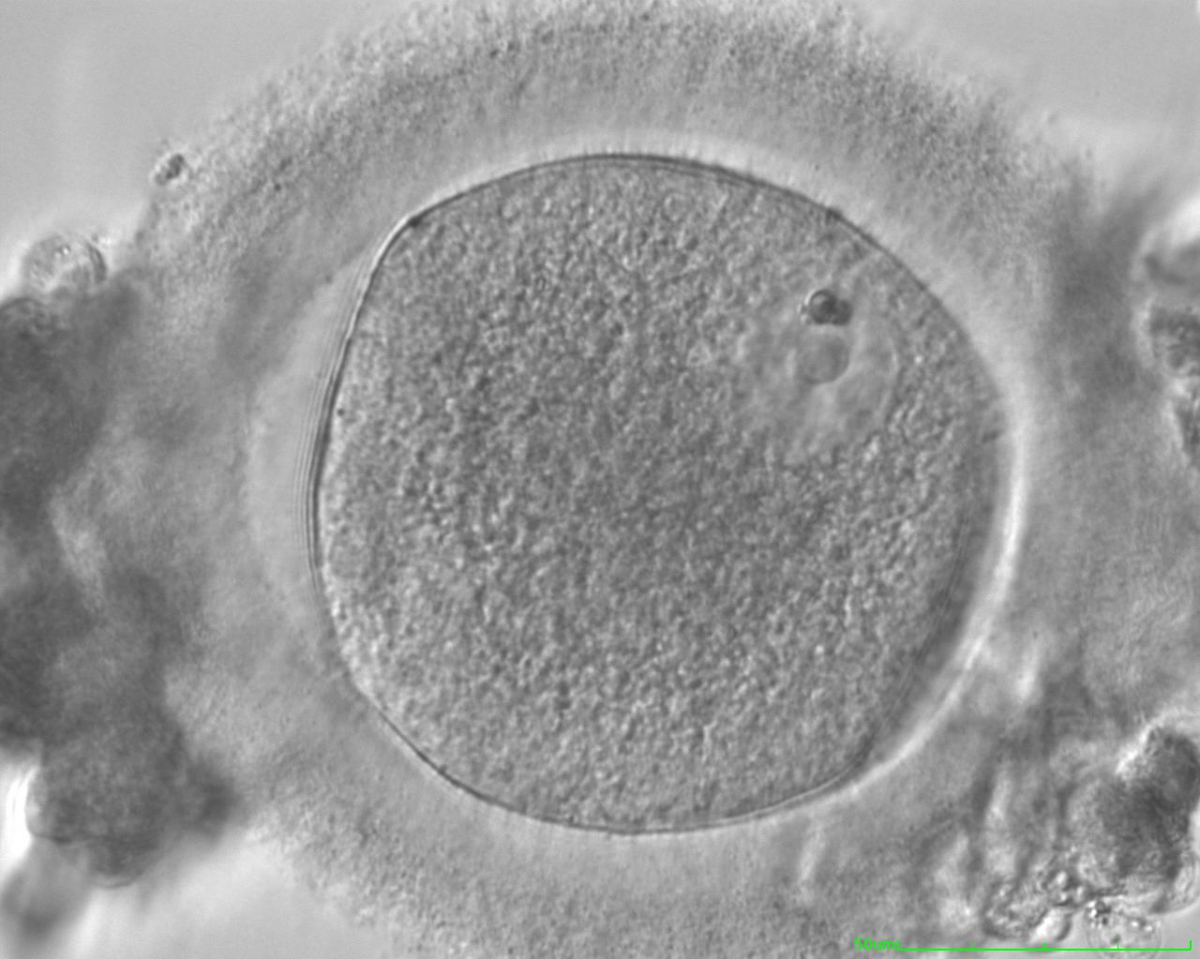
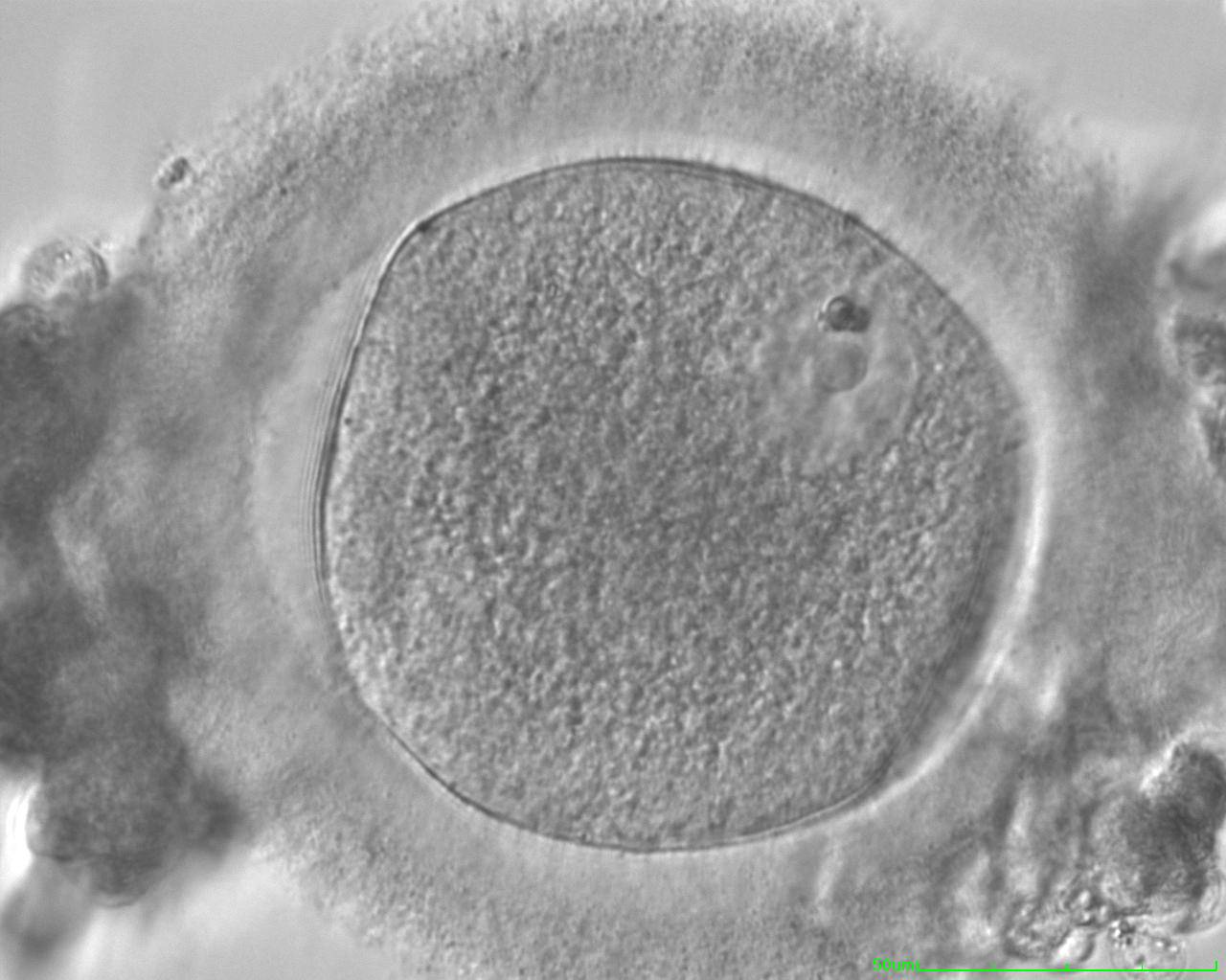
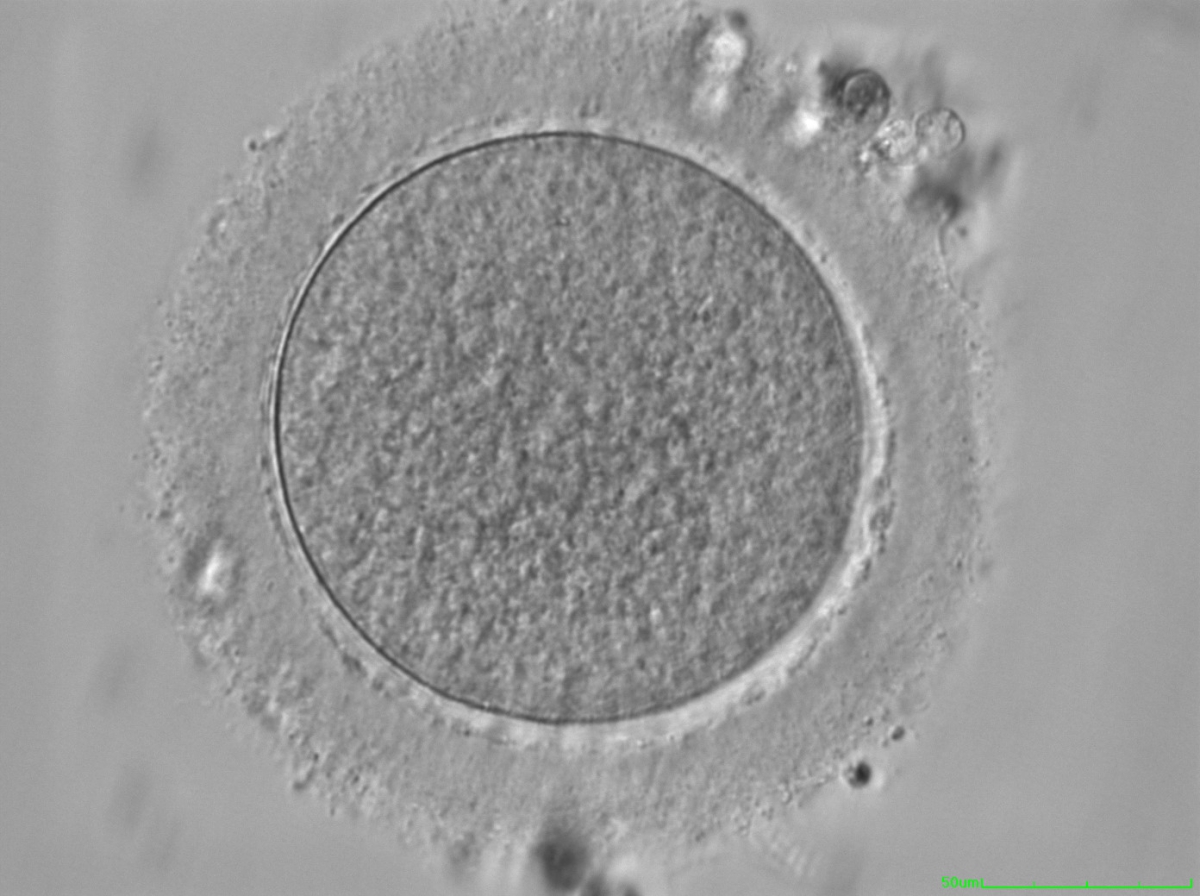
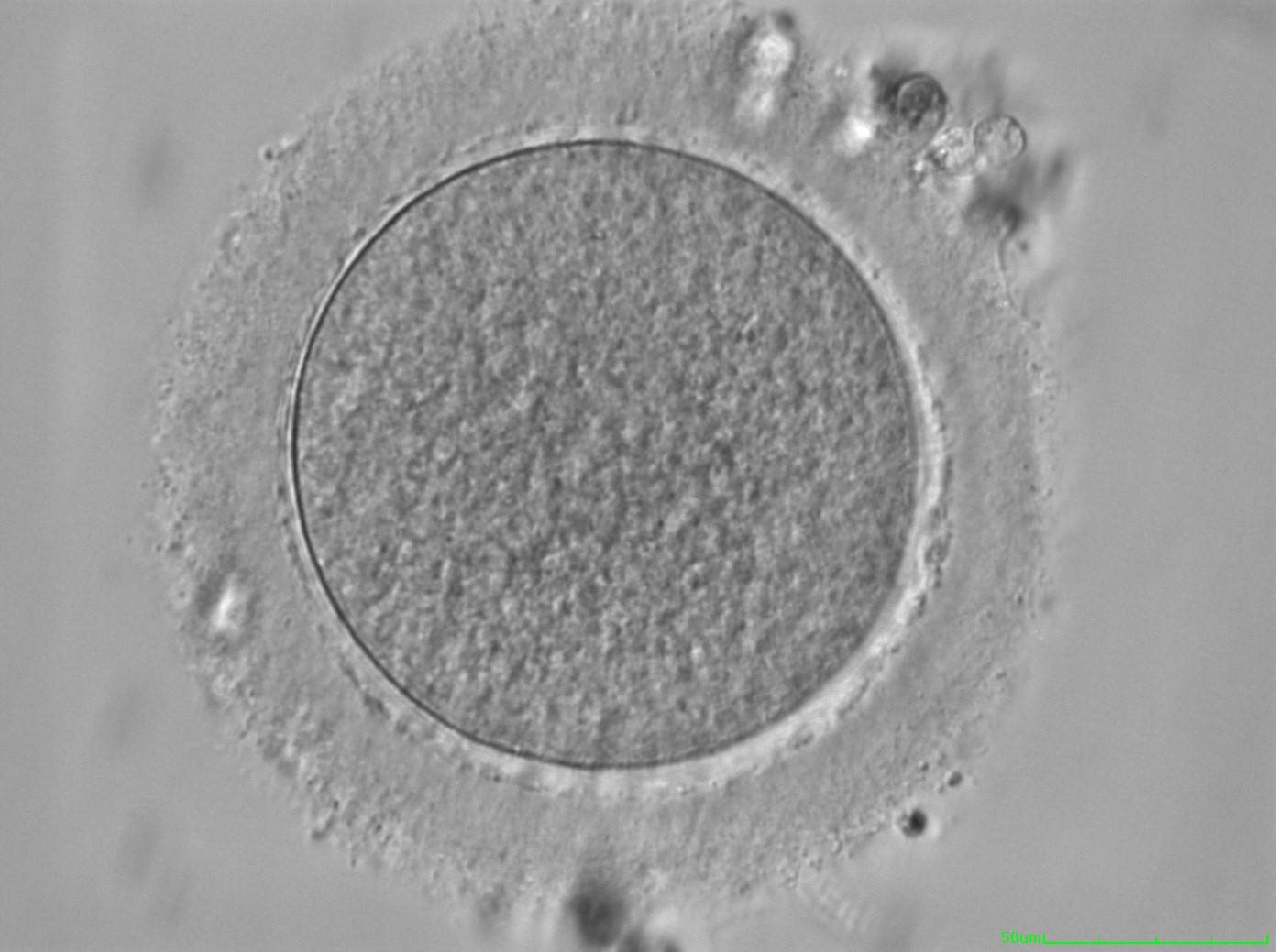
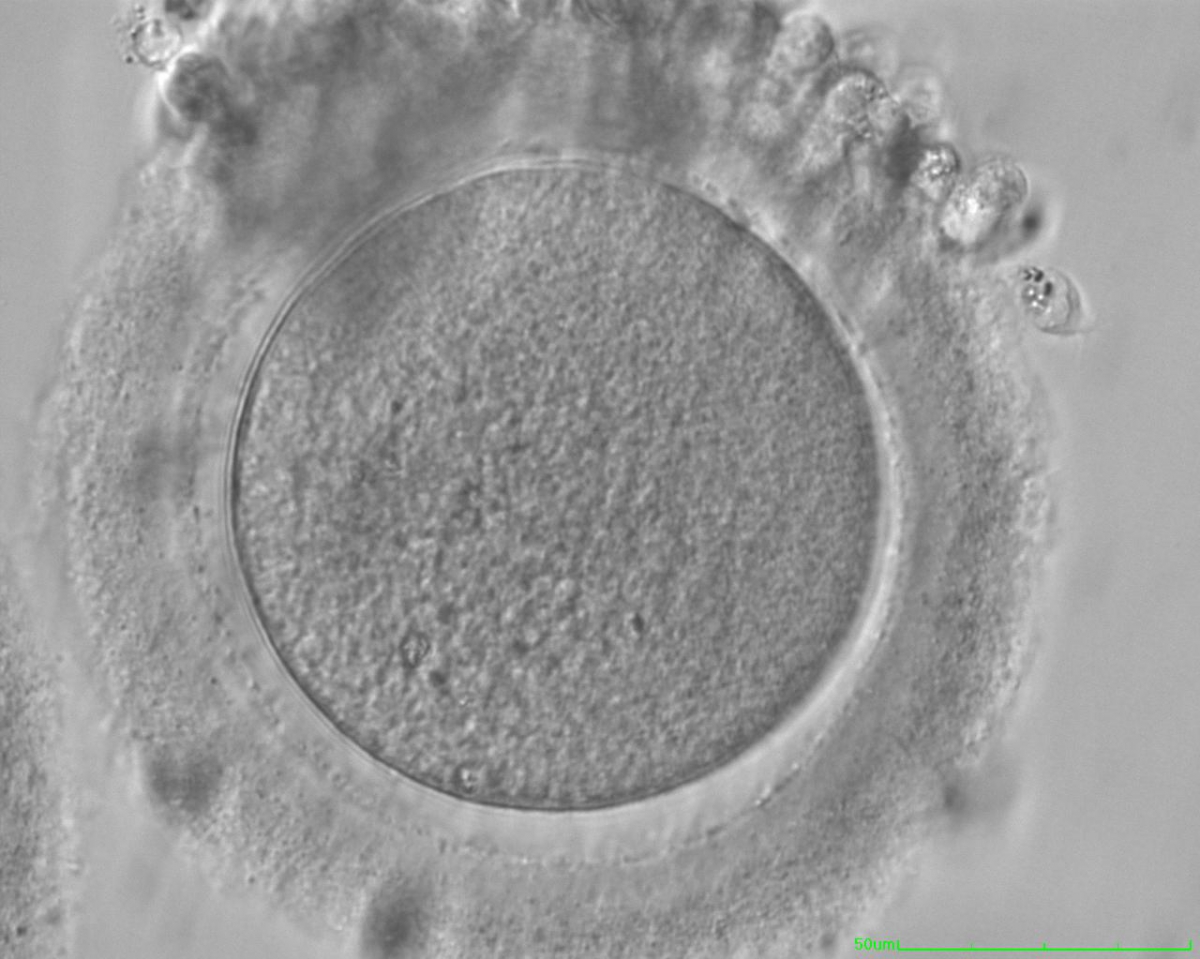
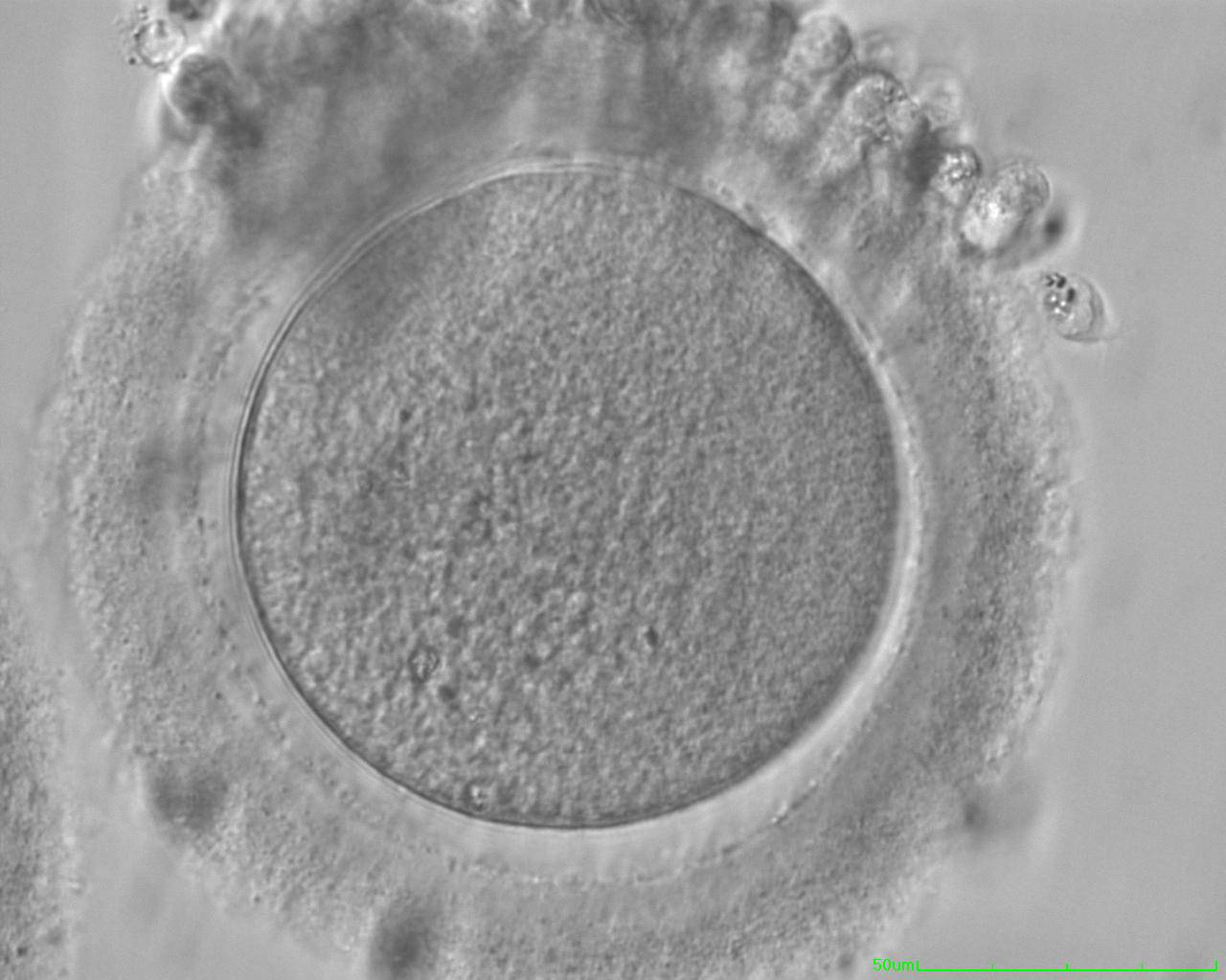
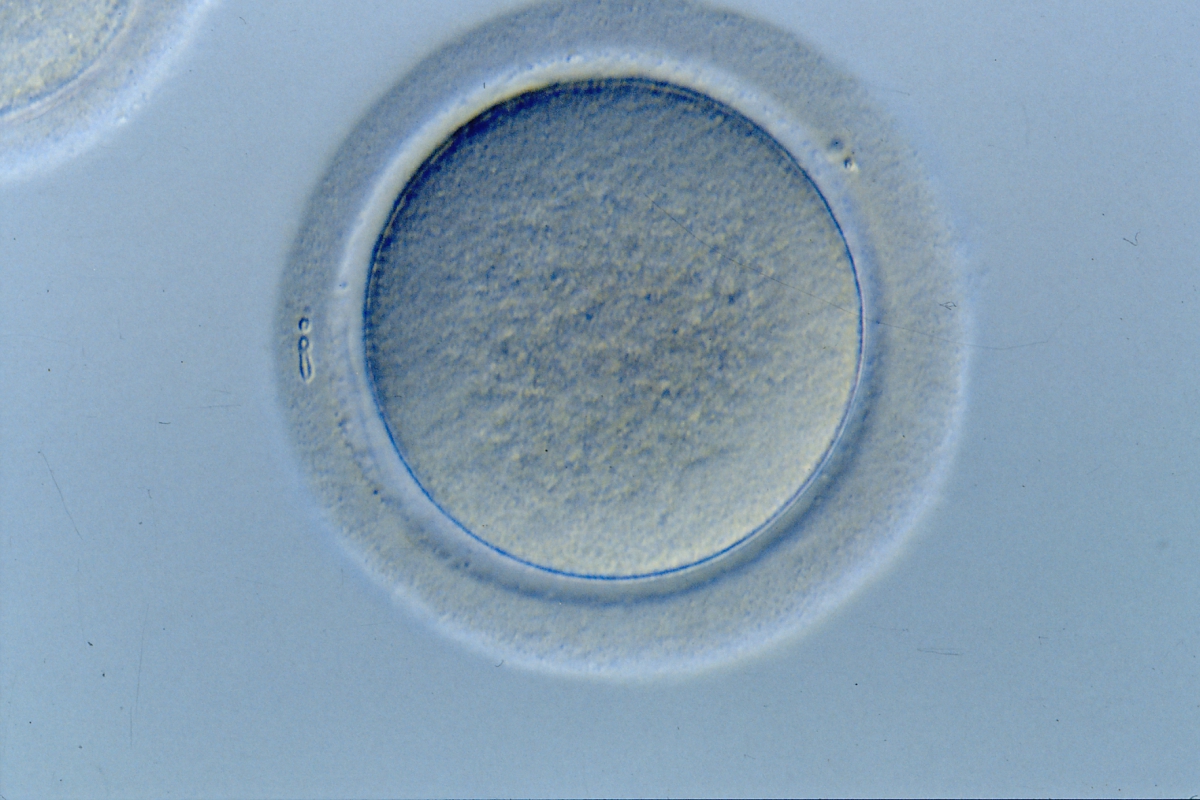
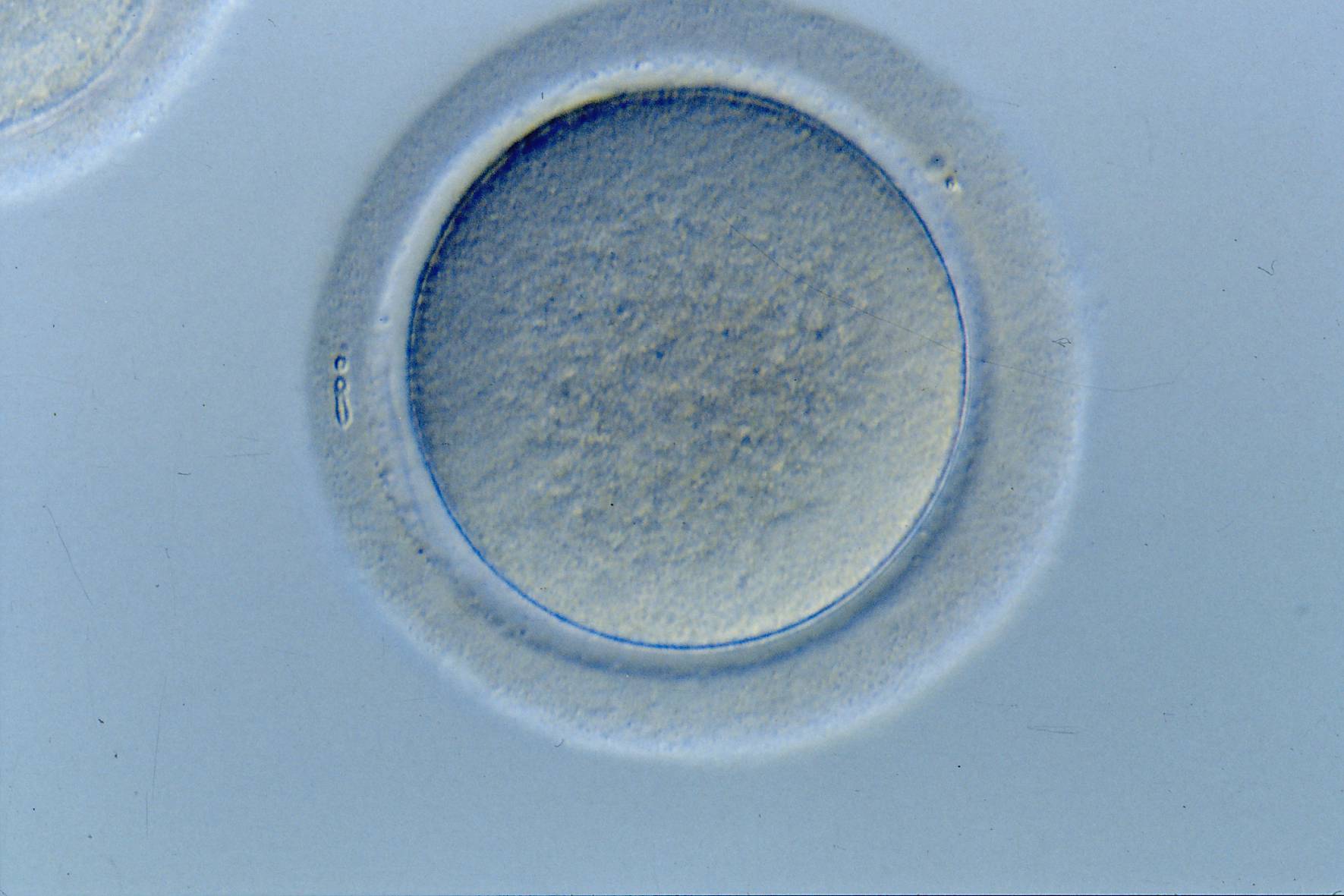
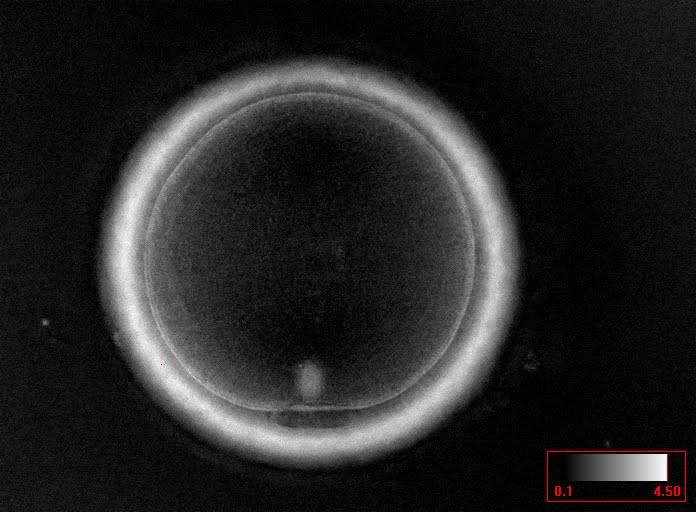
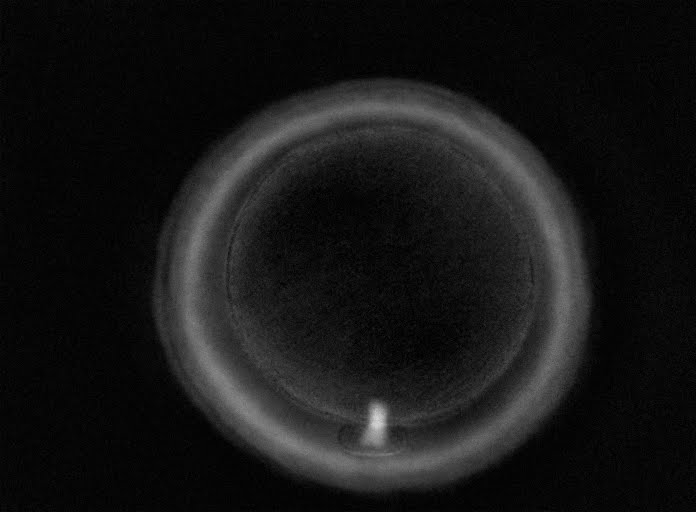
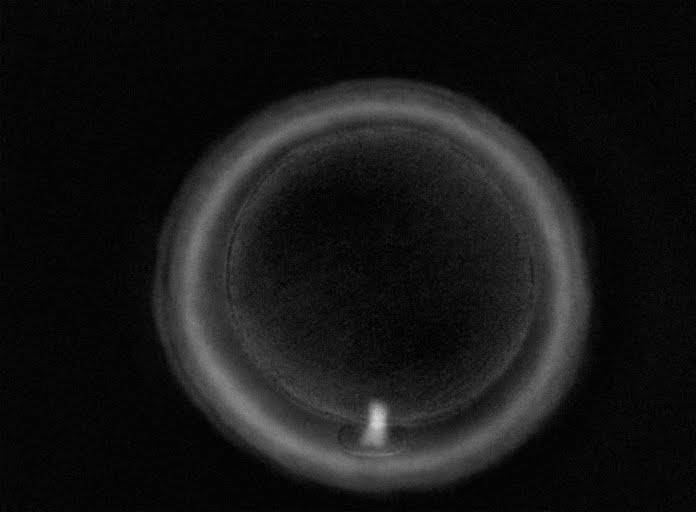
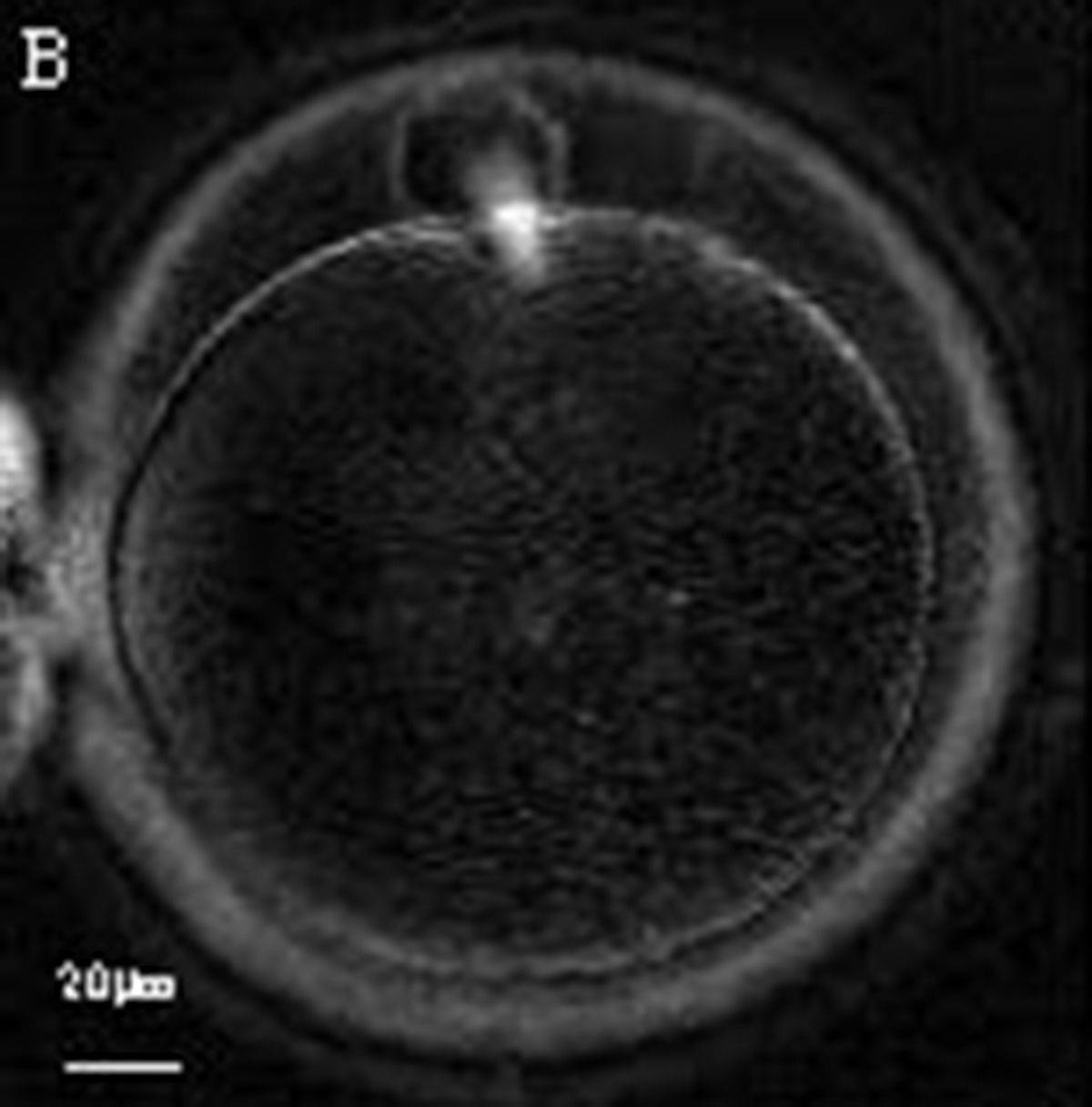
The removal of the cumulus–corona cell mass gives the unique opportunity to evaluate oocyte morphology prior to fertilization, and in particular, the nuclear maturation stage. Oocyte nuclear maturity, as assessed by light microscopy, is assumed to be at the MII stage when the PBI is visible in the PVS (Figs 10 and 11). The MII stage is characterized by the alignment of the homologous chromosomes on the spindle equatorial plate during metaphase of the second meiotic division. It is generally recognized that 85% of the retrieved oocytes following ovarian hyperstimulation display the PBI and are classified as MII, whereas 10% present an intracytoplasmic nucleus called the ‘germinal vesicle’ (GV; Figs 12–14), characteristic of prophase I of the first meiotic division. Approximately 5% of the oocytes have neither a visible GV nor PBI and these oocytes are generally classified as MI oocytes (Figs 15–17; Rienzi and Ubaldi, 2009). These oocytes may, however, be at the GV breakdown stage where the nuclear envelope has broken down but has not as yet progressed to true MI where the chromosomes are aligned on the metaphase plate in preparation for the completion of the first meiotic division.
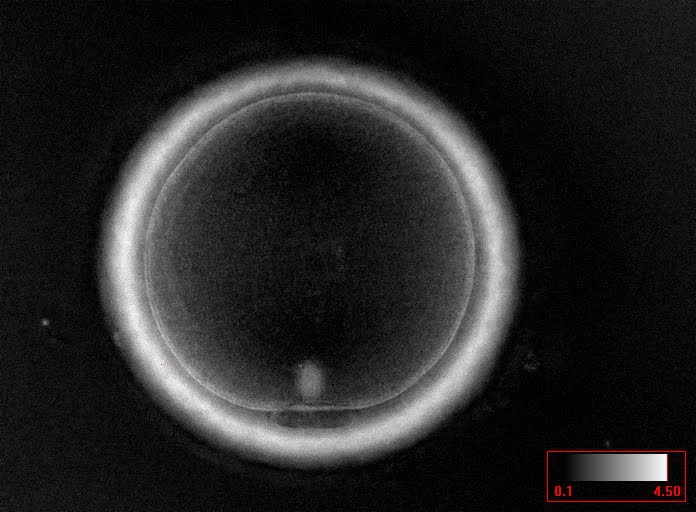
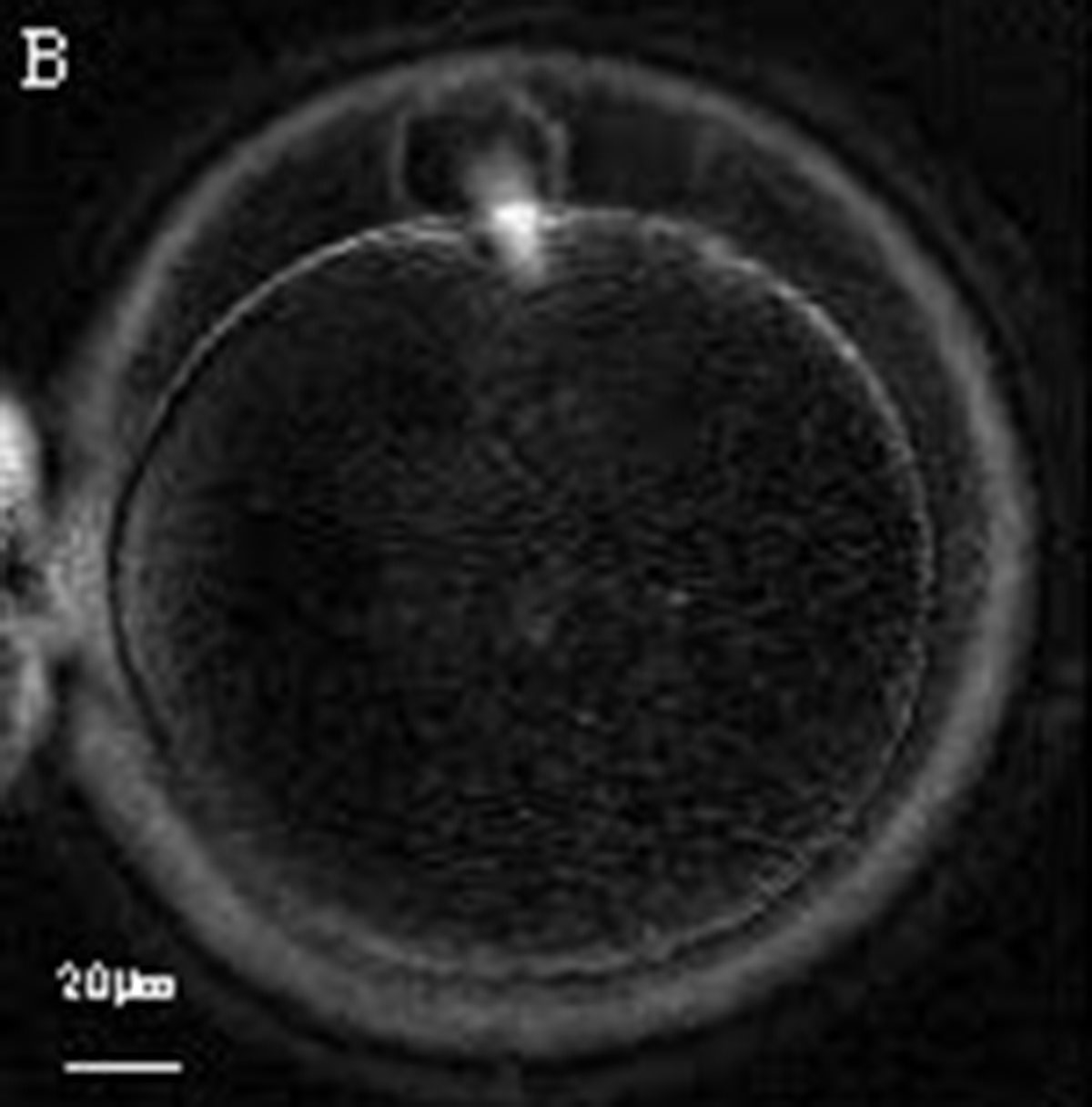
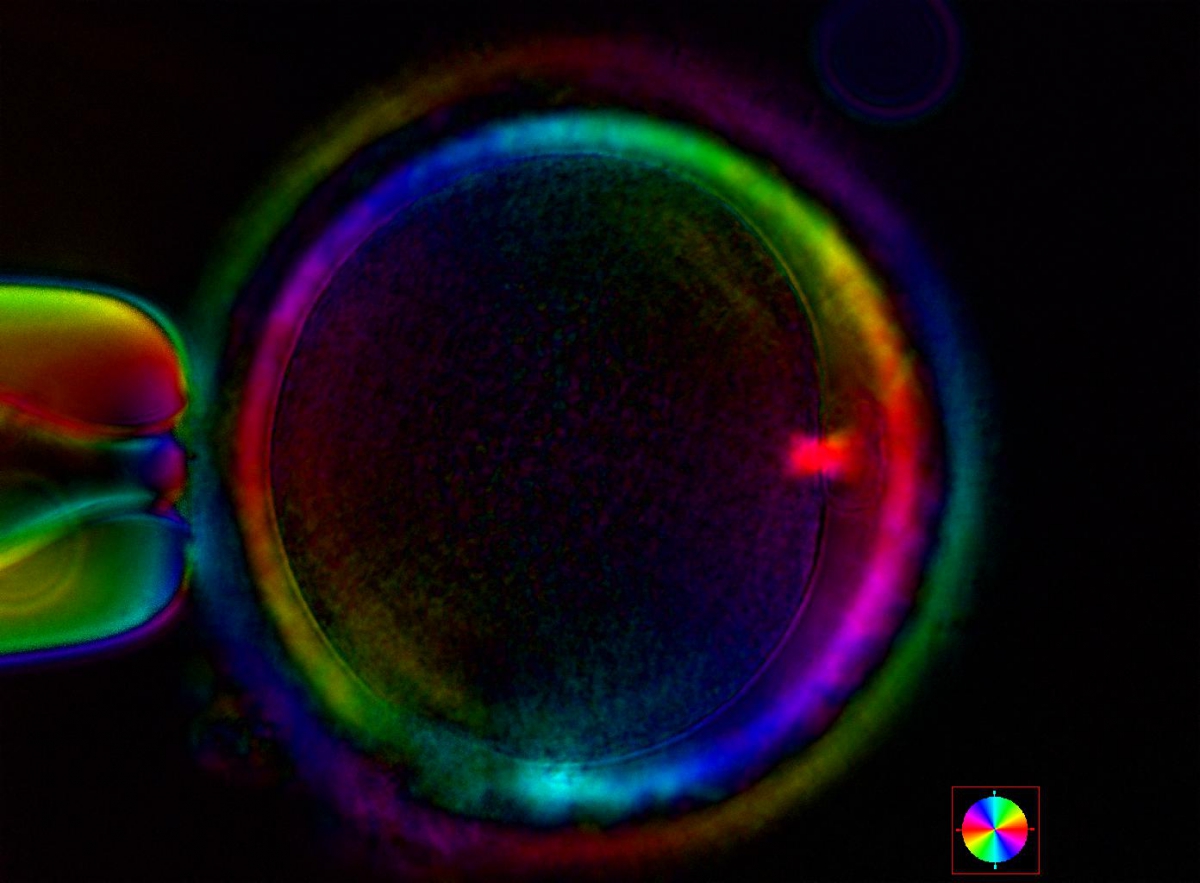
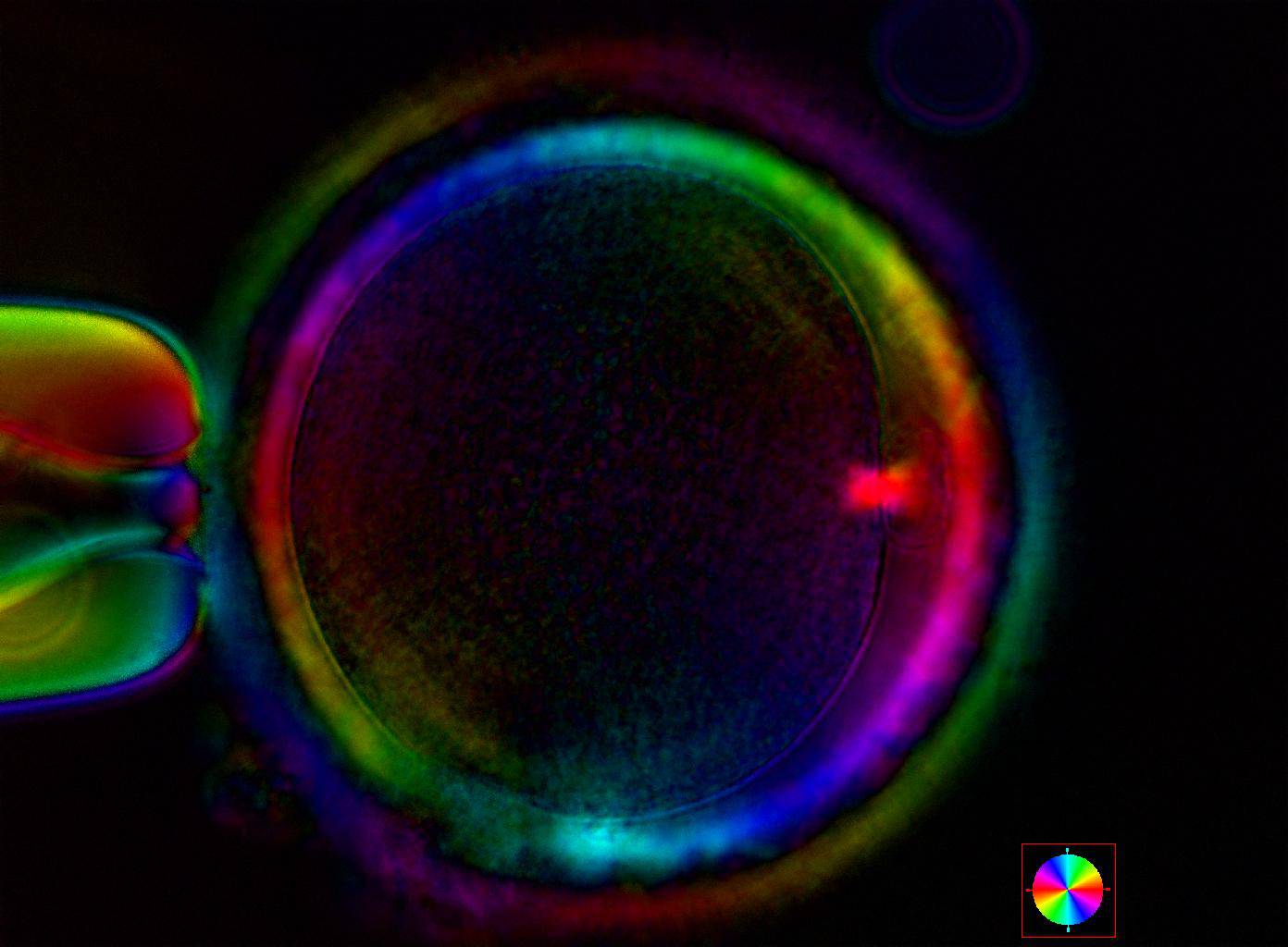
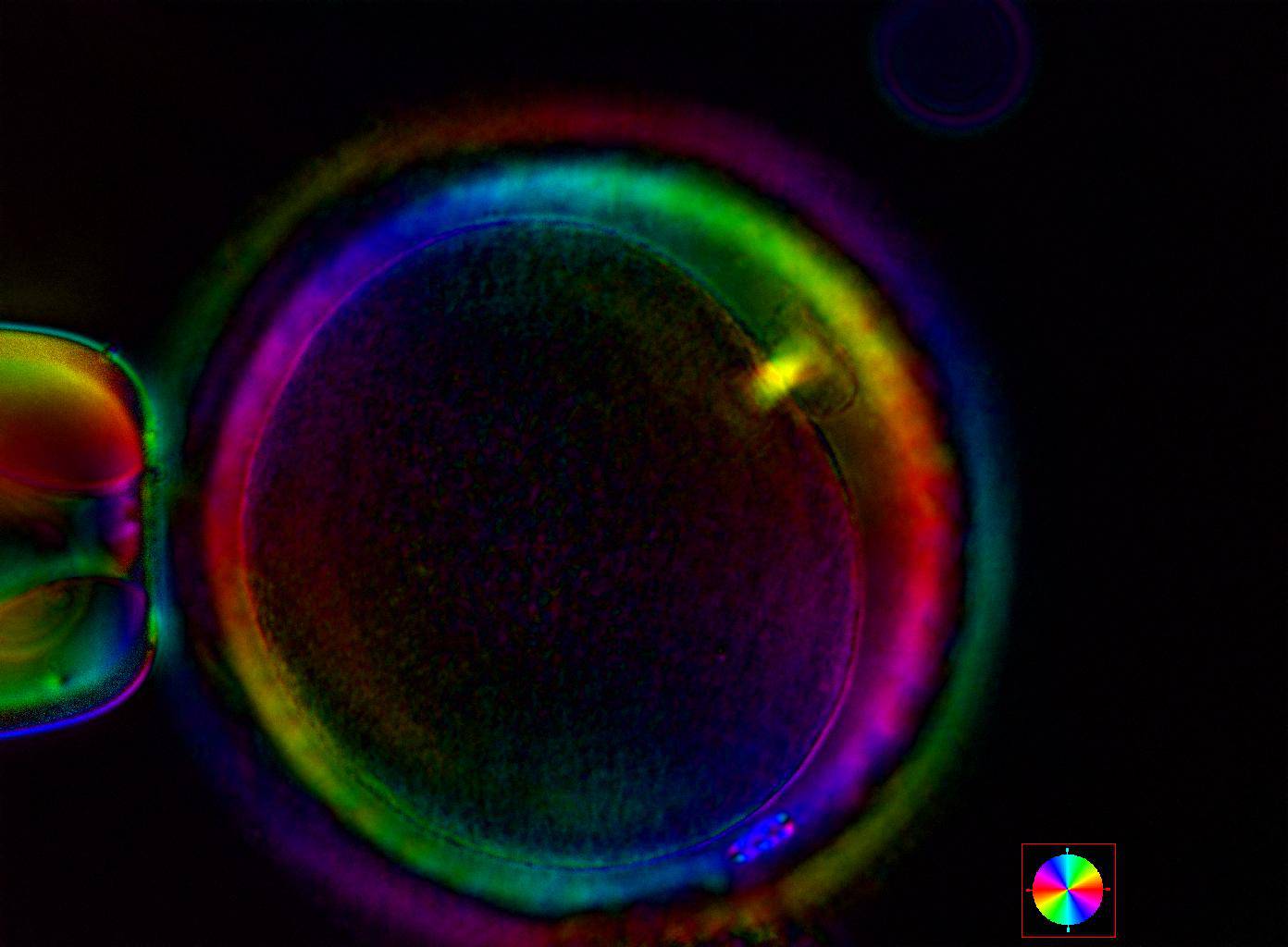
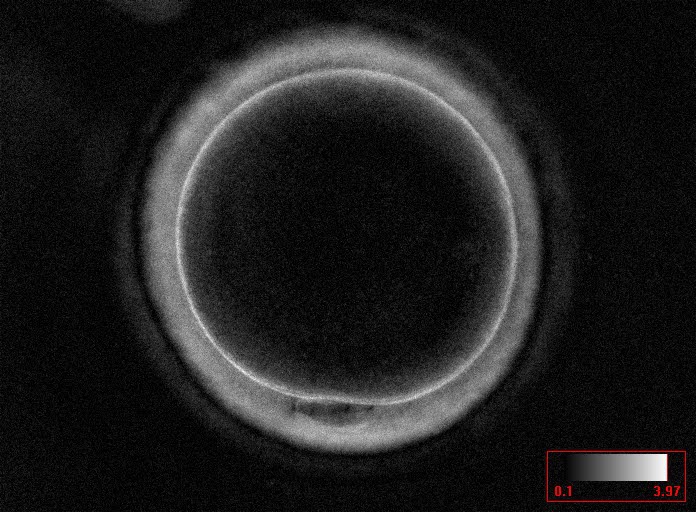
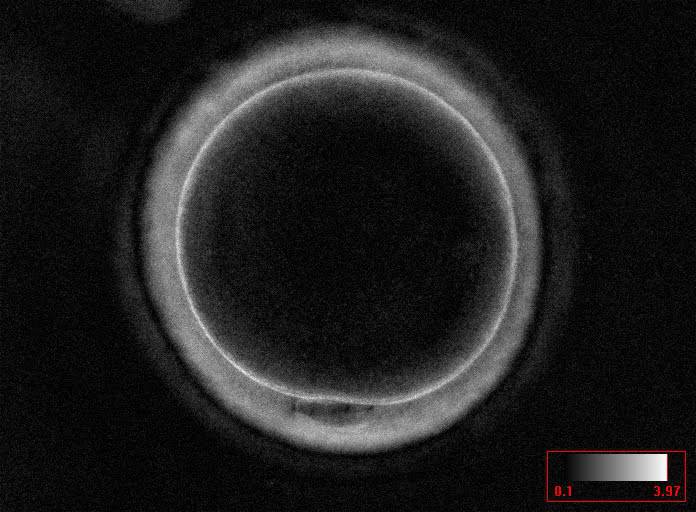
Additional information on oocyte nuclear status can be obtained with the use of polarized light microscopy combined with software for image processing for the non-invasive visualization of the MS and other oocyte birefringent structures. The MS is a microtubular structure involved in chromosome segregation, and therefore is crucial in the sequence of events leading to the correct completion of meiosis and subsequent fertilization. Parallel-aligned MS microtubules are birefringent and able to shift the plane of polarized light inducing a retardance; these properties enable the system to generate contrast and image the MS structure (Oldenbourg, 1999; Fig. 18). The presence of the MS gives more accurate information about the nuclear stage of the oocyte. In particular, some oocytes can be immature (at the stage of early telophase I) when observed with polarized light microscopy, despite the presence of PBI in the PVS. At this stage, in fact, there is continuity between the ooplasm of the oocyte and the forming PBI and the MS is interposed between the two separating cells (Figs 19–22). This condition normally has a duration of 75–90 min. The MS has been found to disappear in late telophase I (Fig. 23), reforming only 40–60 min later (Montag et al., 2011). However, it must be underlined that other factors, such as sub-optimal culture conditions, temperature fluctuations and chemical stress during manipulation, can contribute to MS disassembly (Rienzi and Ubaldi, 2009). Finally, the percentage of oocytes with detectable MS is also related to the time elapsed from HCG administration and is higher after 38 h (Cohen et al., 2004). In general, it is expected that at least 80% of oocytes recovered following ovarian hyperstimulation are MS positive when viewed by polarized light microscopy.
Article references:
Cohen Y, Malcov M, Schwartz T, Mey-Raz N, Carmon A, Cohen T, Lessing JB, Amit A, Azem F. Spindle imaging: a new marker for optimal timing of ICSI? Hum Reprod 2004;19:649-654.
Abstract/FREE Full Text
Oldenbourg R. Polarized light microscopy of spindles. Methods Cell Biol 1999;61:175-208.
Medline | Web of Science | Google Scholar
Rienzi L, Ubaldi F. Oocyte retrieval and selection. In: Gardner DK, Weissman A, Howles CM, Shoham Z, editors. Textbook of Assisted Reproductive Technologies: Laboratory and Clinical Perspectives. 3rd edn. London, UK: Informa Healthcare; 2009. p. 5-101.
Google Scholar
Montag M, Köster M, Van der Ven K, van der Ven H. Gamete competence assessment by polarizing optics in assisted reproduction. Hum Reprod Update 2011;17:654-666.
Abstract/FREE Full Text