D. Cytoplasmic features
D.1 Ooplasm
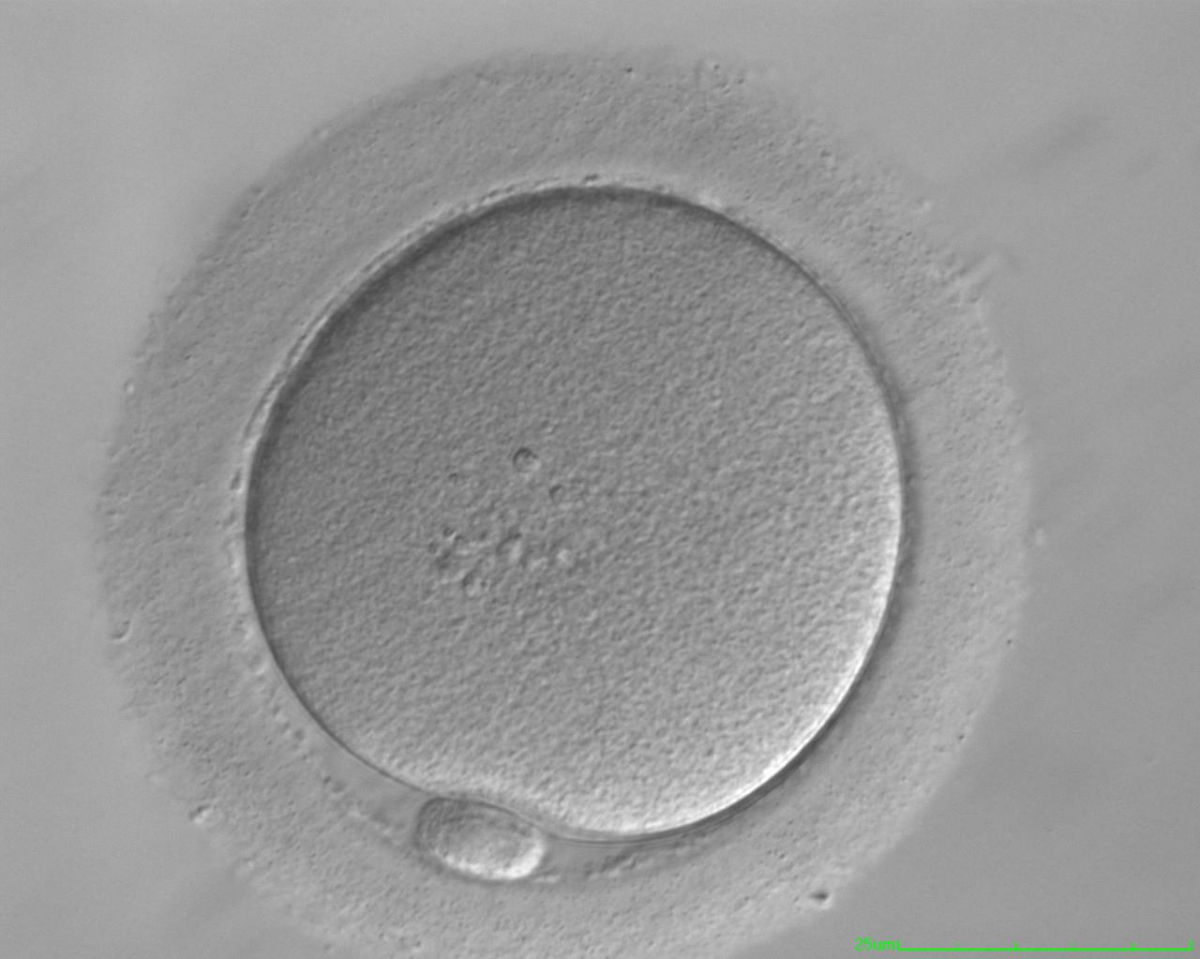
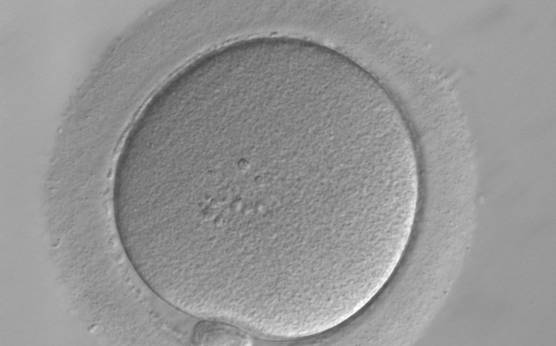
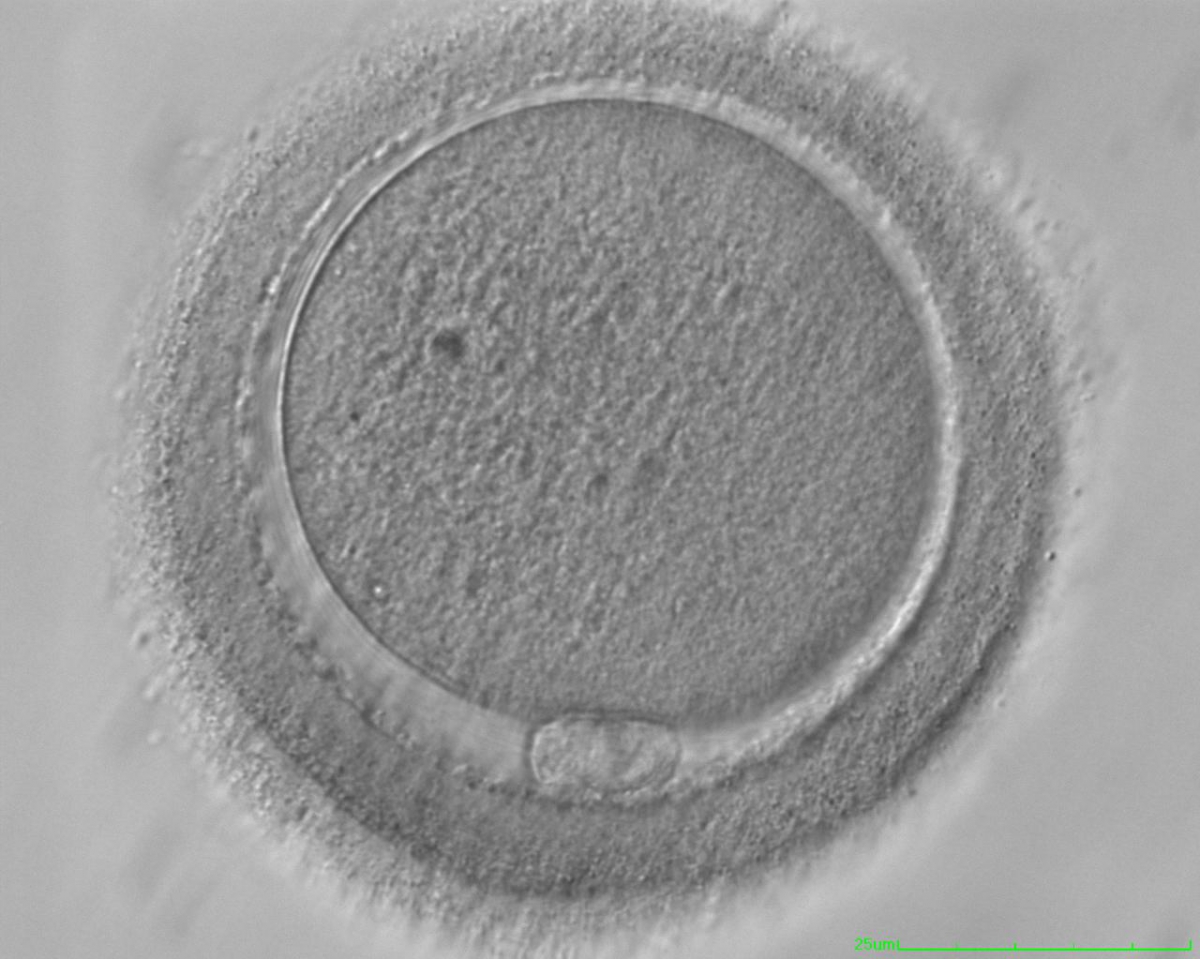
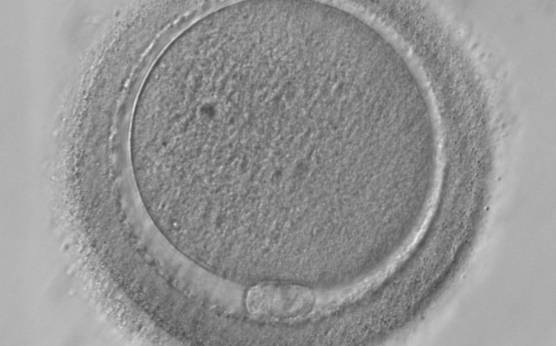
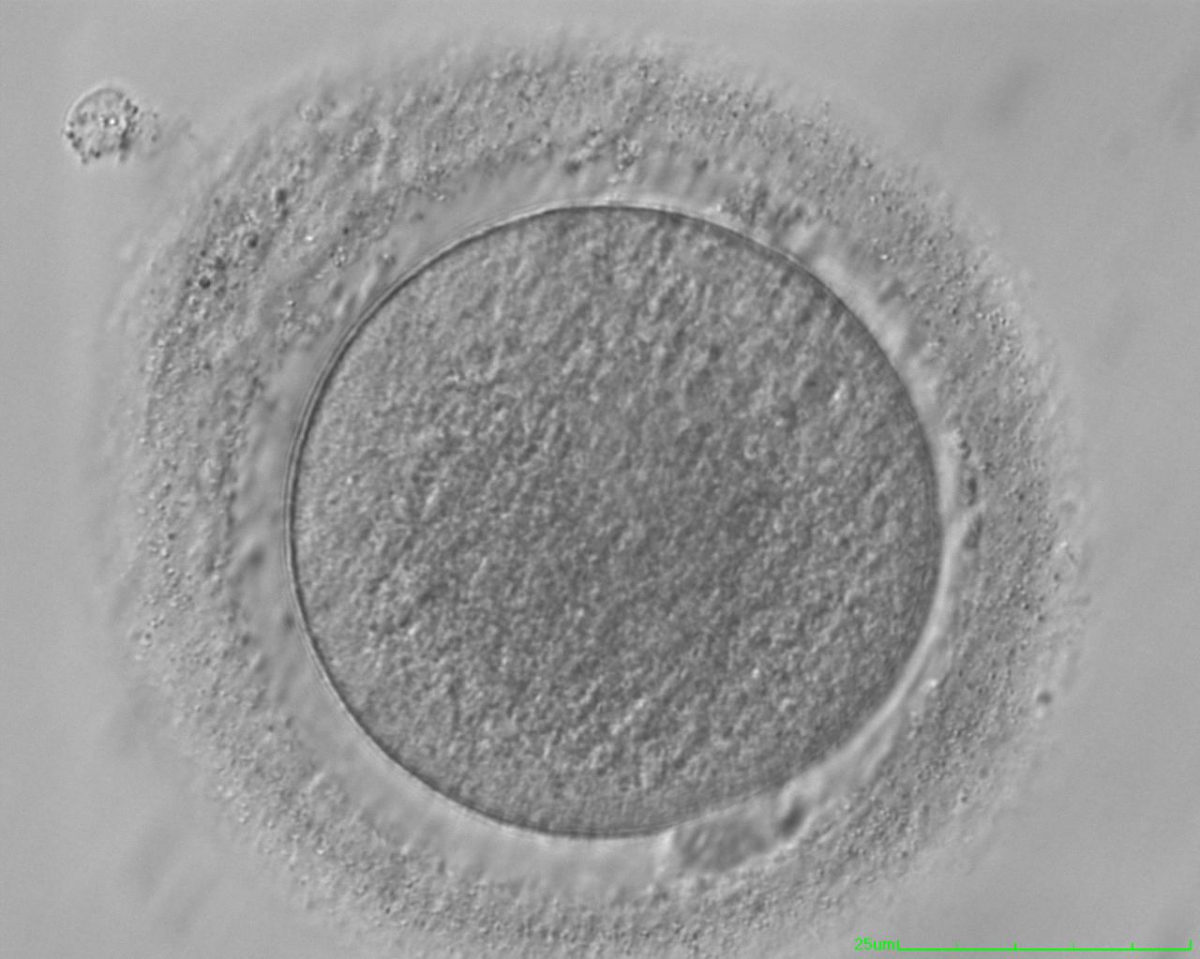
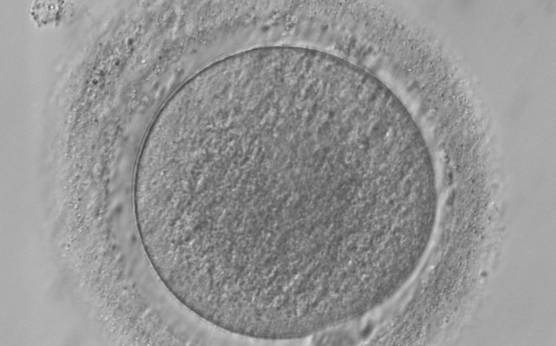
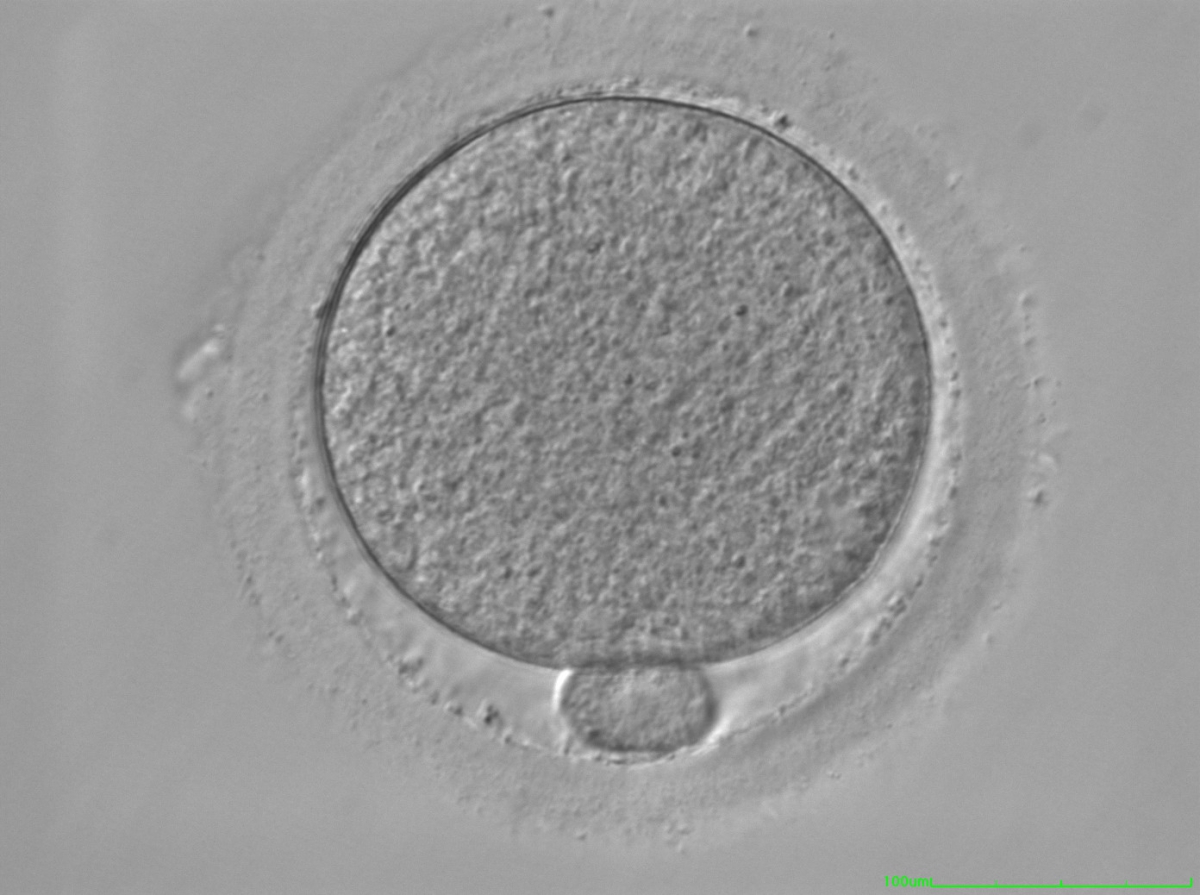
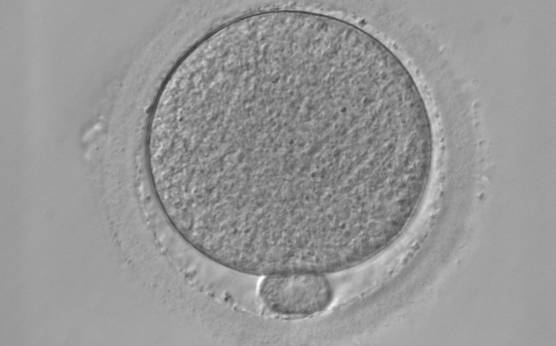
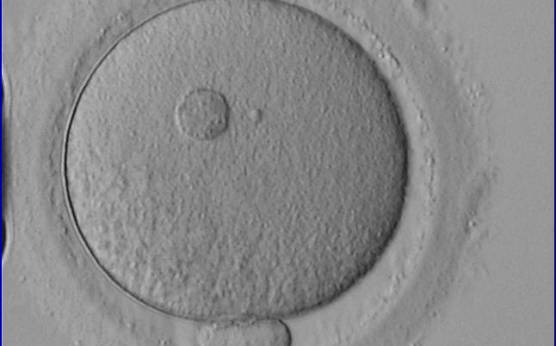
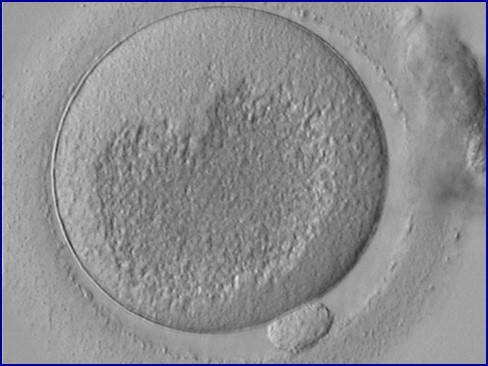
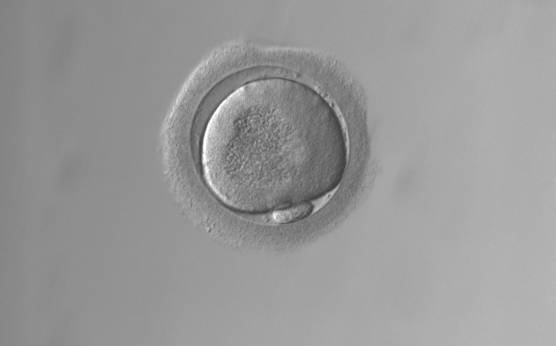
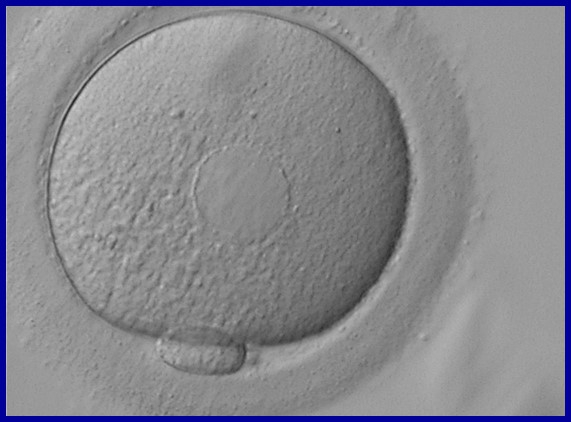
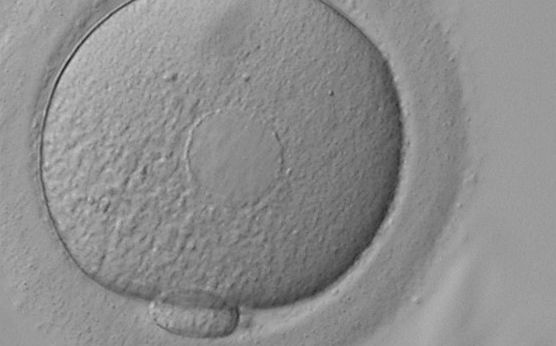
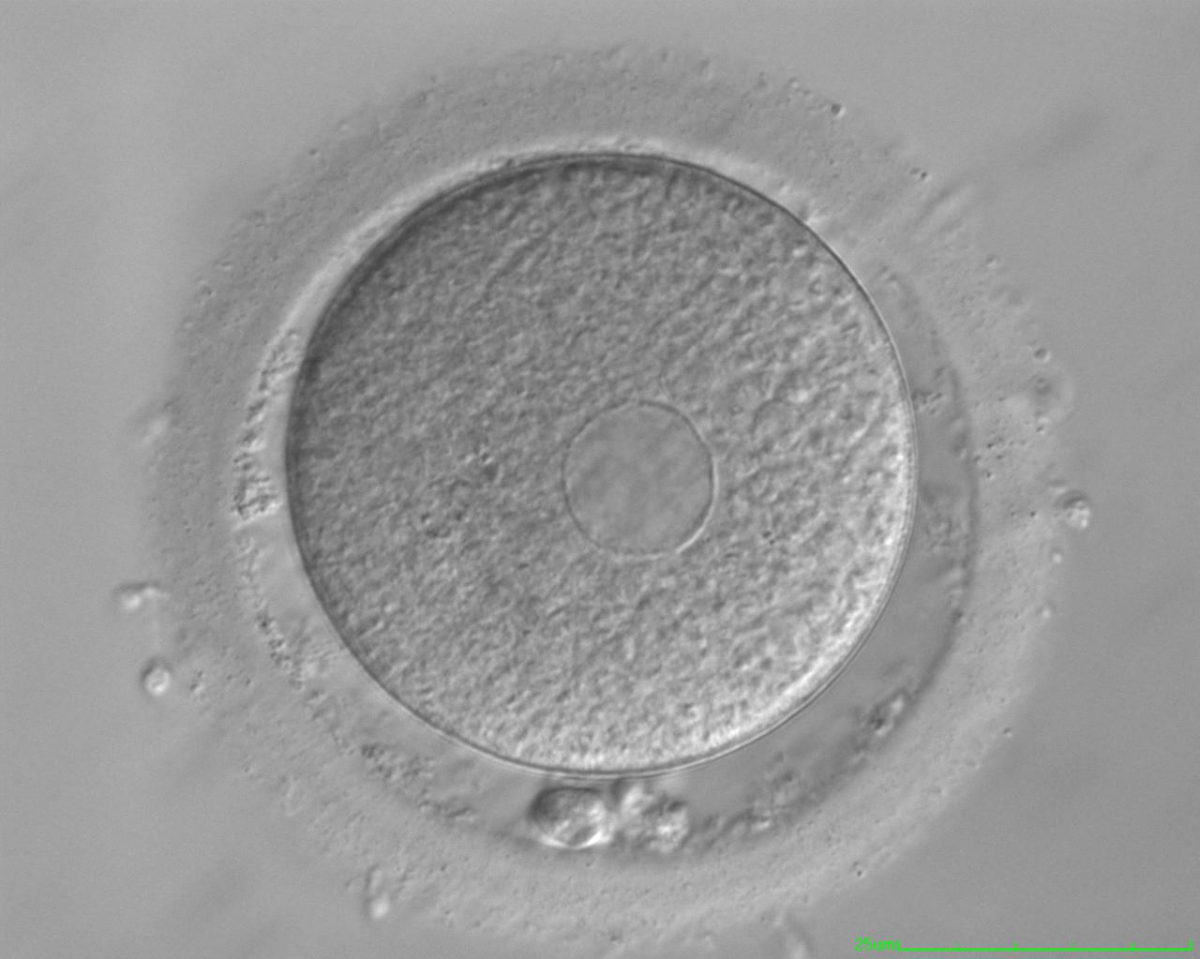
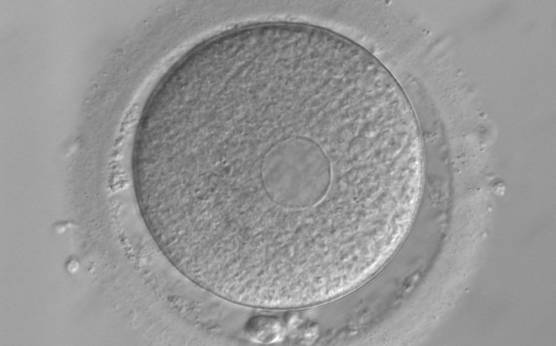
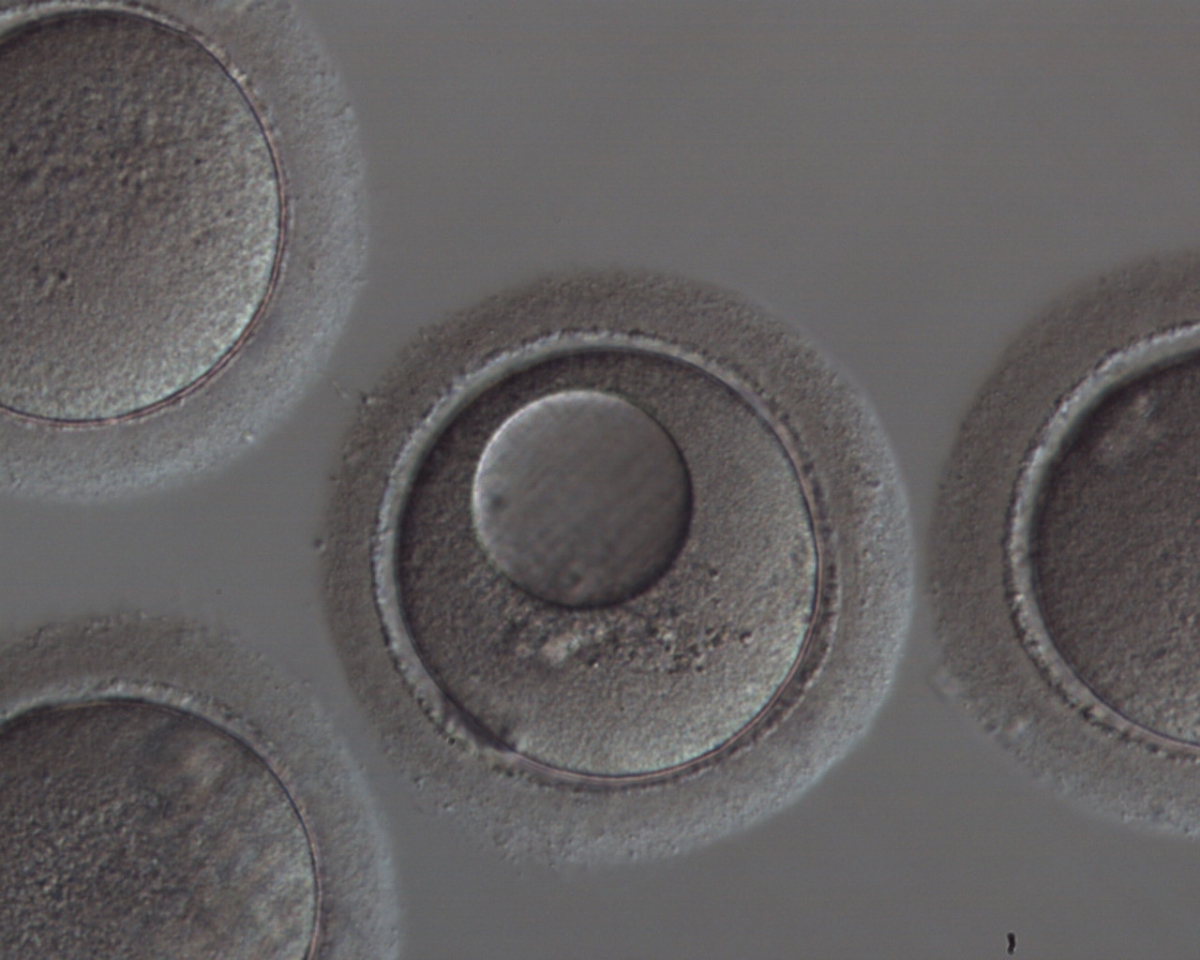
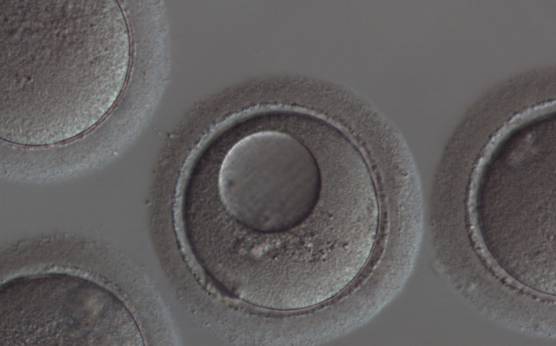
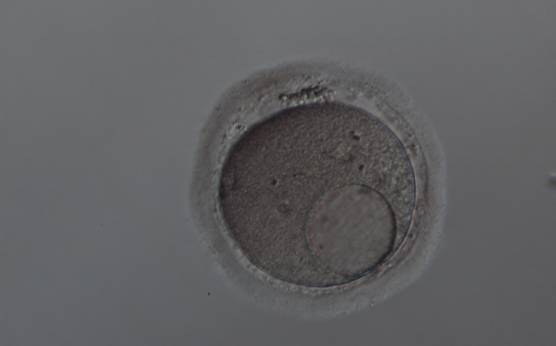
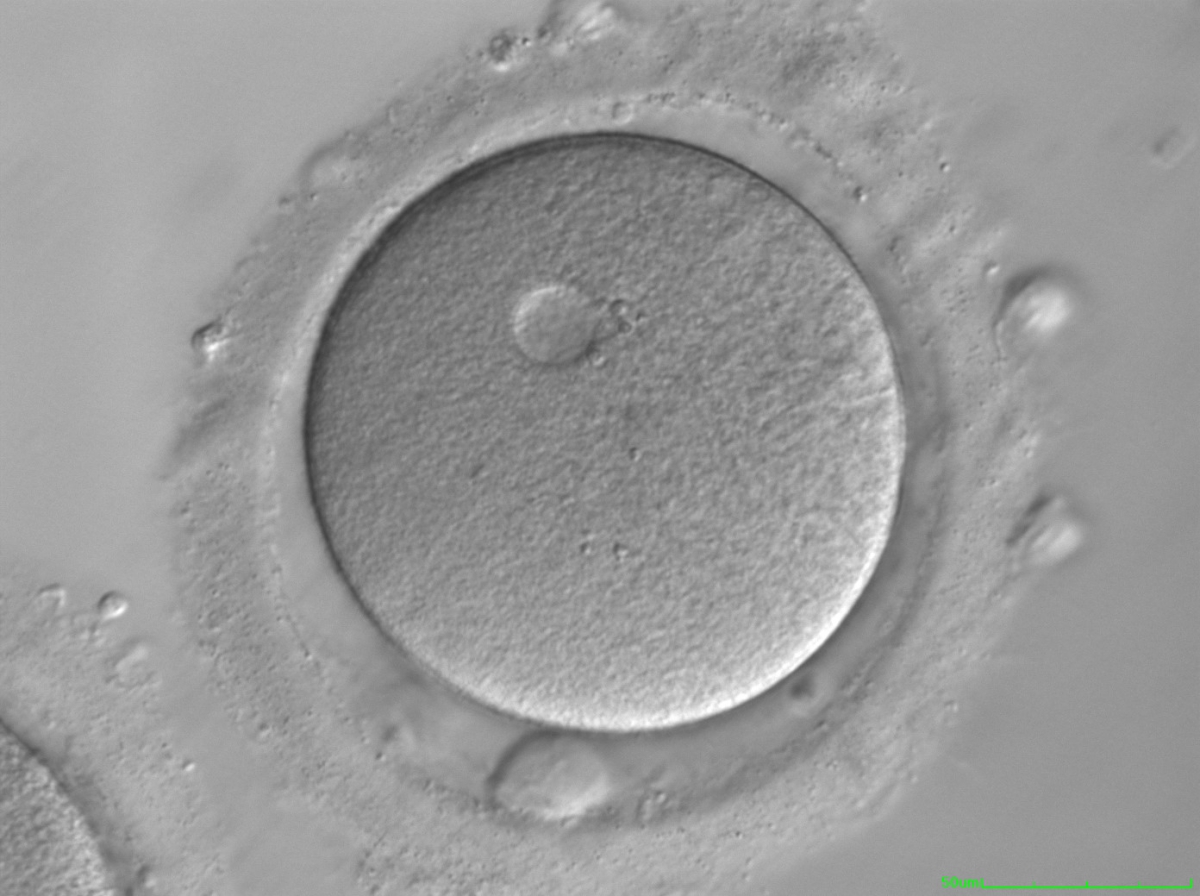
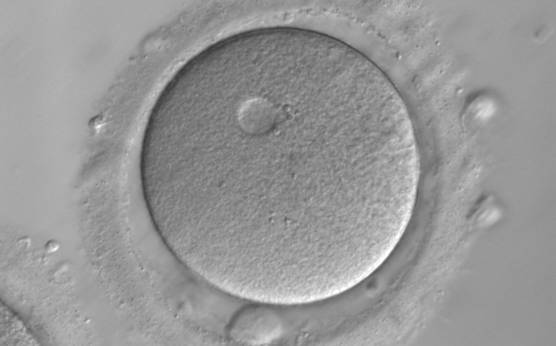
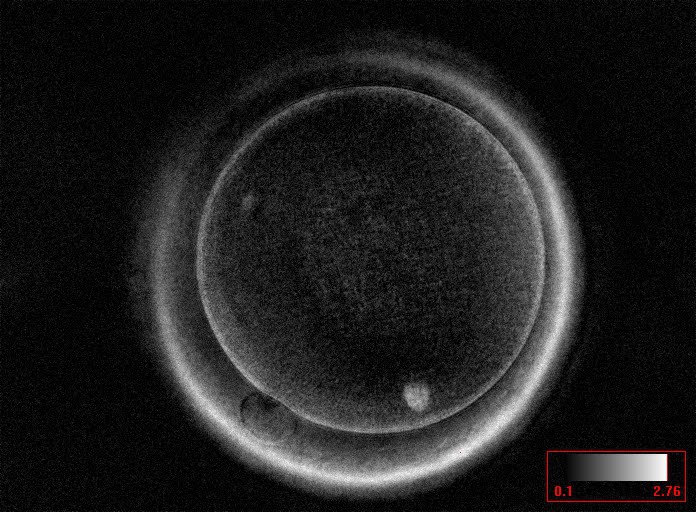
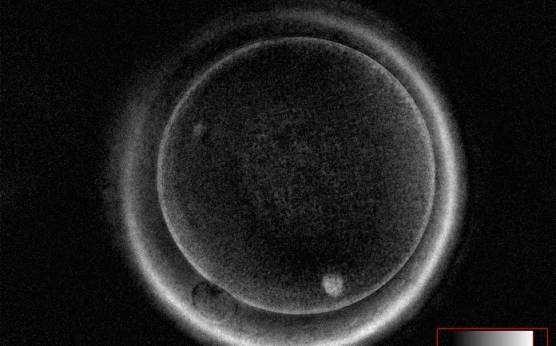
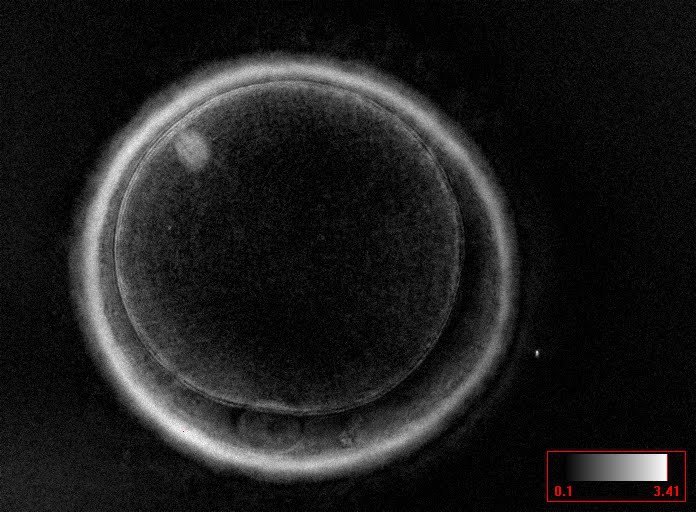
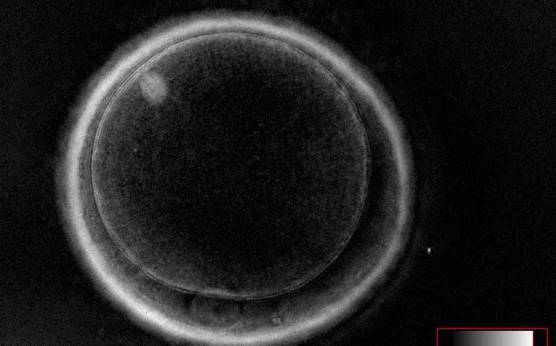
It has been shown in the literature that severe dysmorphisms of the cytoplasmic texture impairs the developmental and implantation potential of the embryo (Balaban and Urman, 2006). Although a homogeneous, normal cytoplasm is expected in recovered oocytes (Figs 36 and 37), the biological significance of different degrees of heterogeneity in the ooplasm is unknown. Based on current evidence, slightly heterogeneous cytoplasm may only represent normal variability among retrieved oocytes rather than being an abnormality of developmental significance (Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology, 2011).
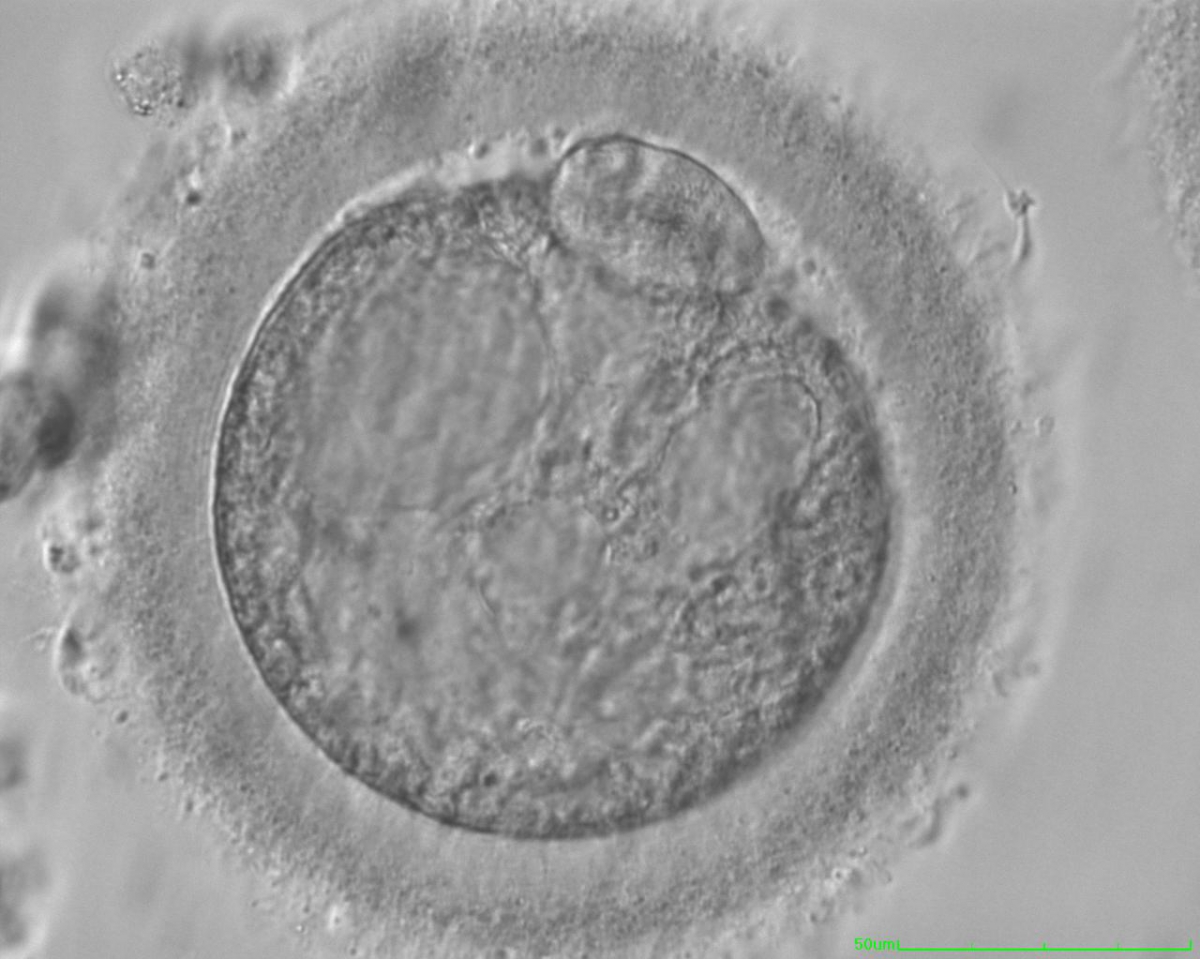
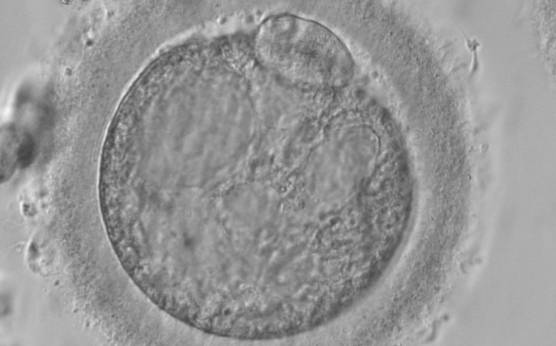
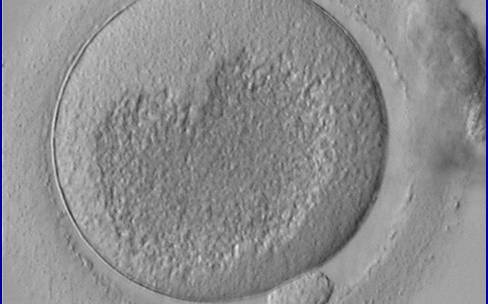
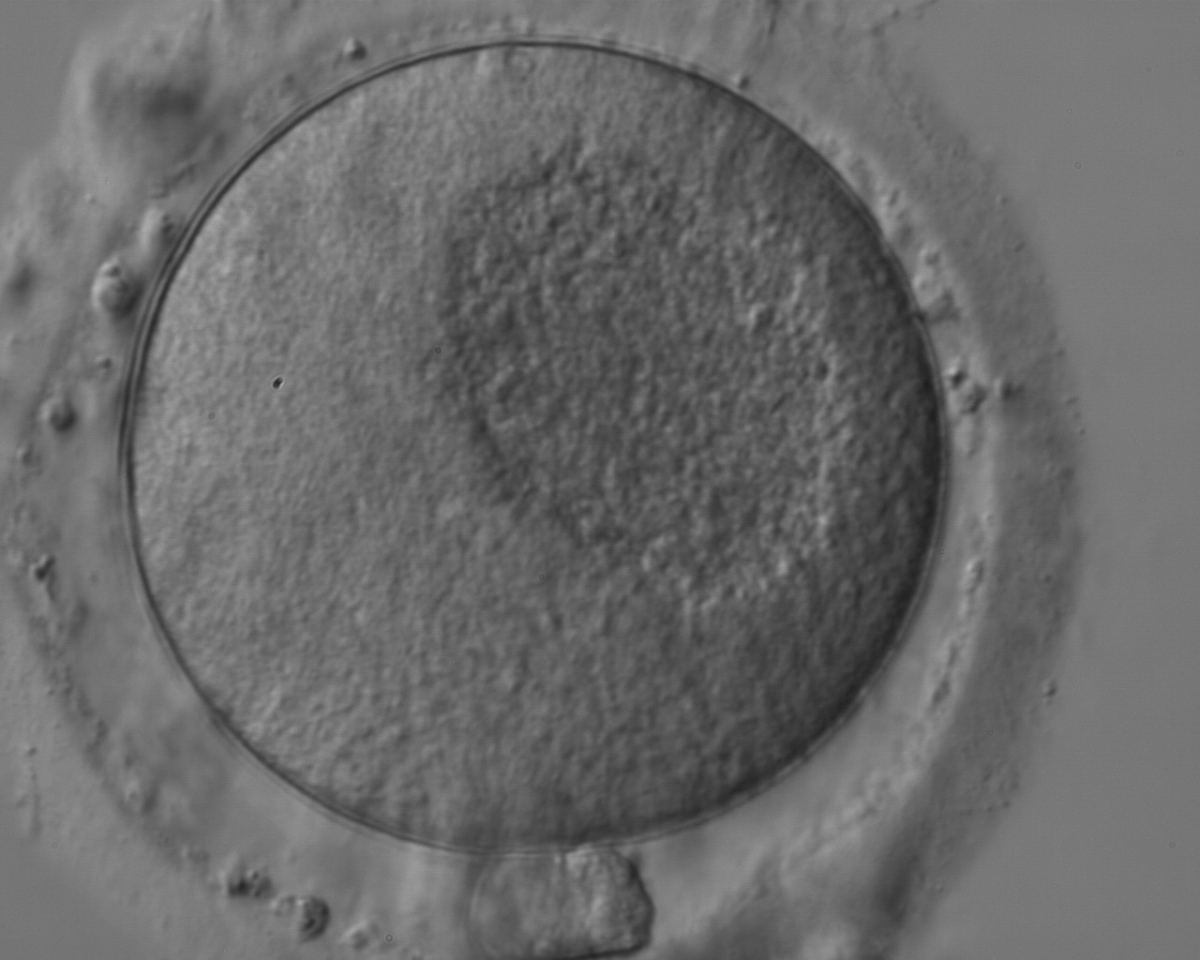
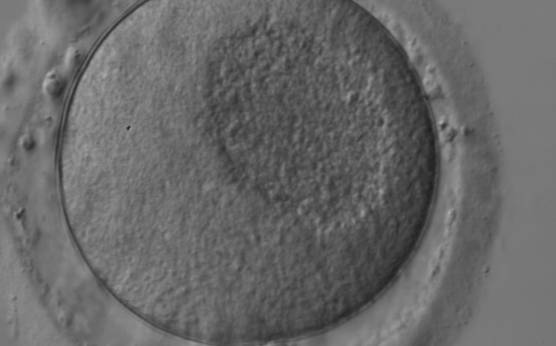
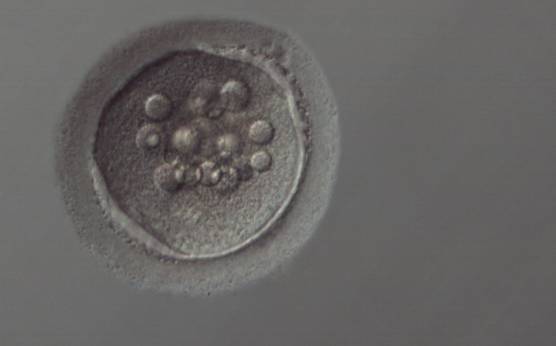
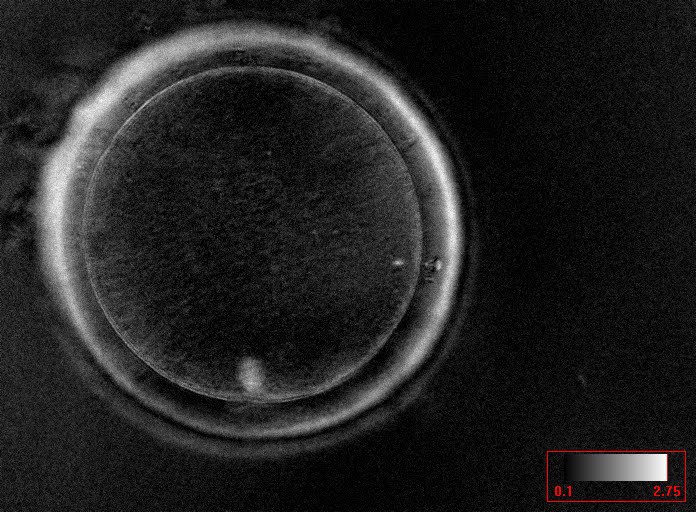
Granularities of the cytoplasm (Figs 38–41) are poorly defined in the literature and may depend on the modulation of the optical path used in phase contrast microscopy. These morphological deviations should be carefully distinguished from inclusions such as refractile bodies or lipofuscin bodies (Otsuki et al., 2007; Fig. 42) and organelle clustering which is also described as very condensed centralized granularity detectable by any form of microscopy (Figs 43–45; Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology, 2011).
One of the most important severe cytoplasmic abnormalities of MII oocytes is the appearance of SER clusters (SER discs) within the cytoplasm. SER discs can be identified as translucent vacuole-like structures in the cytoplasm by phase contrast microscopy (Figs 46 and 47). Evidence-based data clearly demonstrates that the embryos derived from oocytes with SER discs are associated with the risk of serious, significantly abnormal outcomes (Balaban and Urman, 2006; Ebner et al., 2006). It is recommended that oocytes with this feature should not be used for injection, and sibling oocytes should be additionally examined for the presence of either a single SER disc or a series of smaller plaques (Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology, 2011).
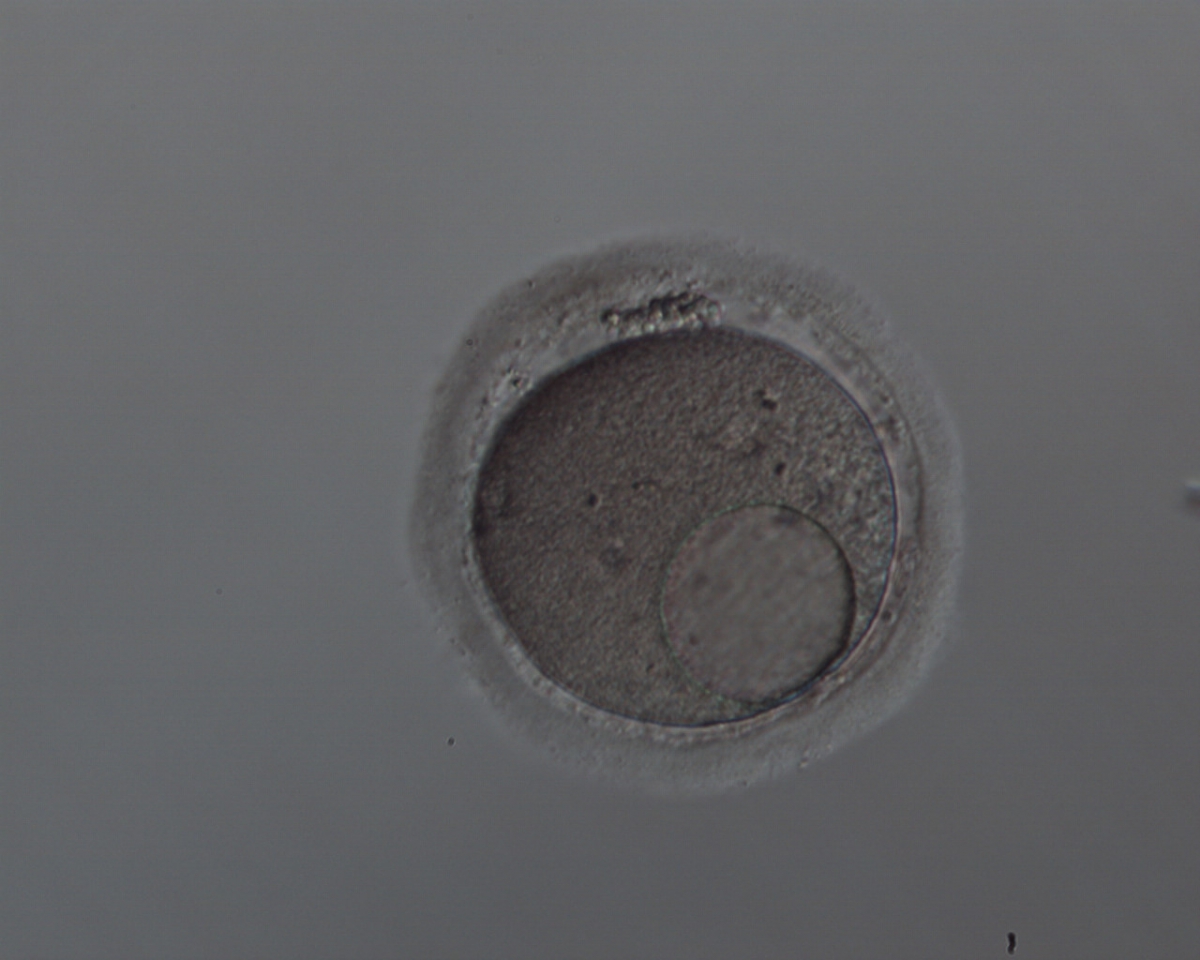
Vacuoles within the cytoplasm are defined as fluid-filled structures which can be more easily noticeable and differ from SER discs (Figs 48–51). Small vacuoles of 5–10 µm in diameter are unlikely to have a biological consequence, whereas large vacuoles >14 µm are associated with fertilization failure. In oocytes that are fertilized, those vacuoles that persist beyond syngamy can interfere with cleavage planes, resulting in a lower blastocyst rate (Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology, 2011).
D.2 Metaphase plate
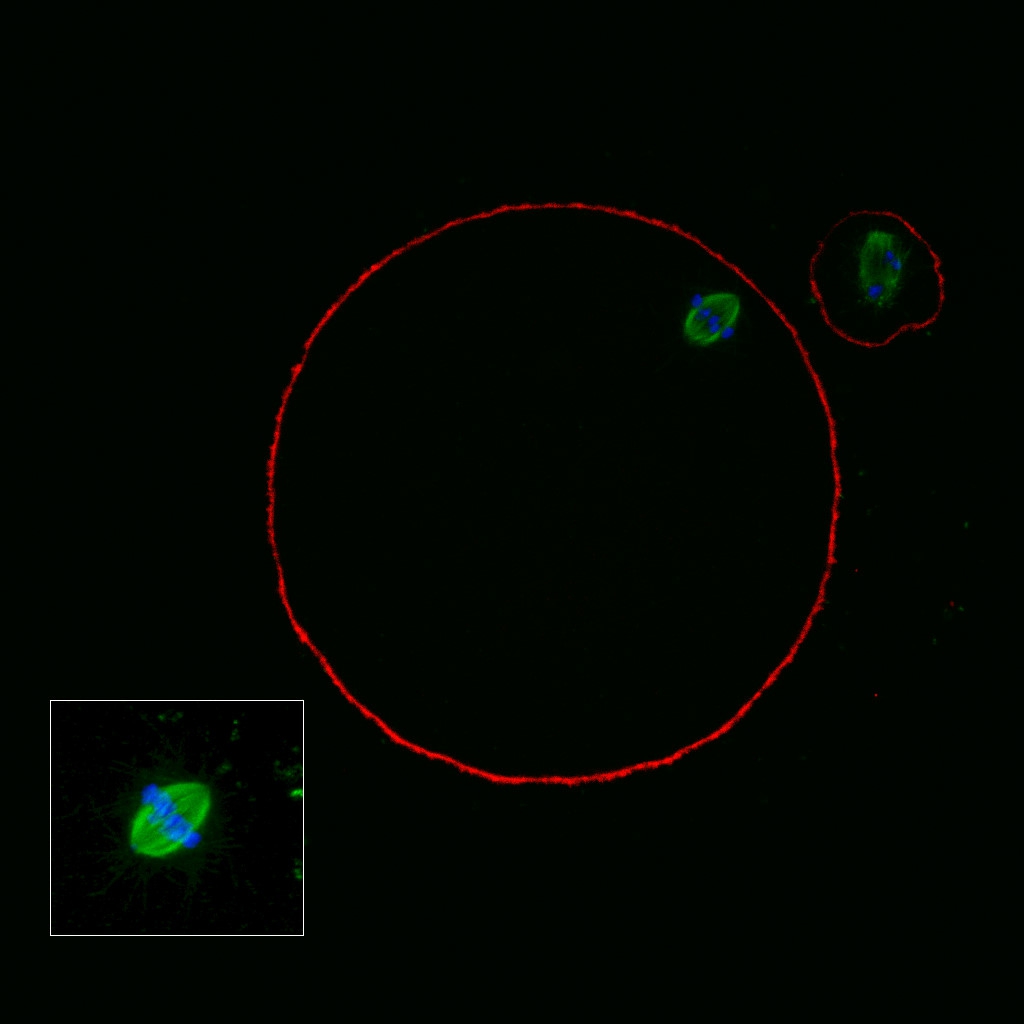
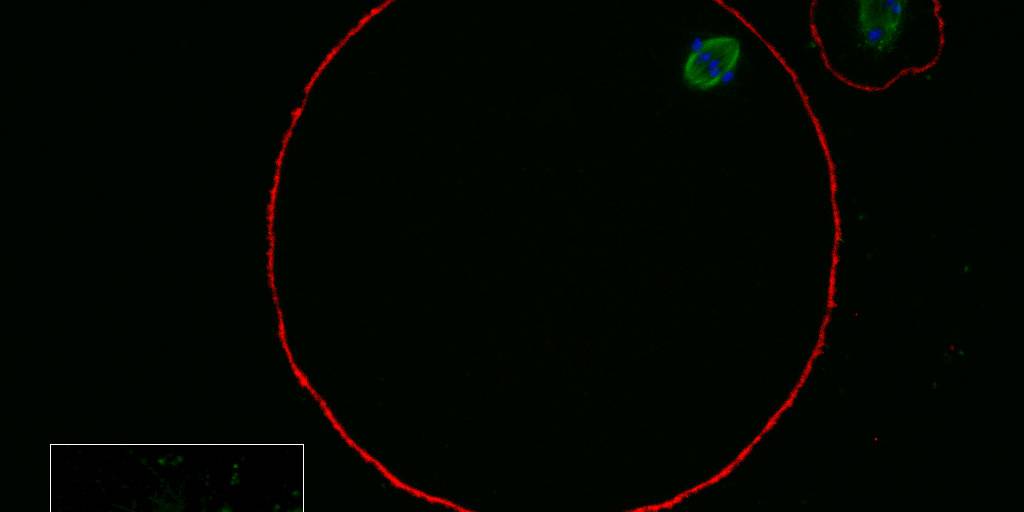
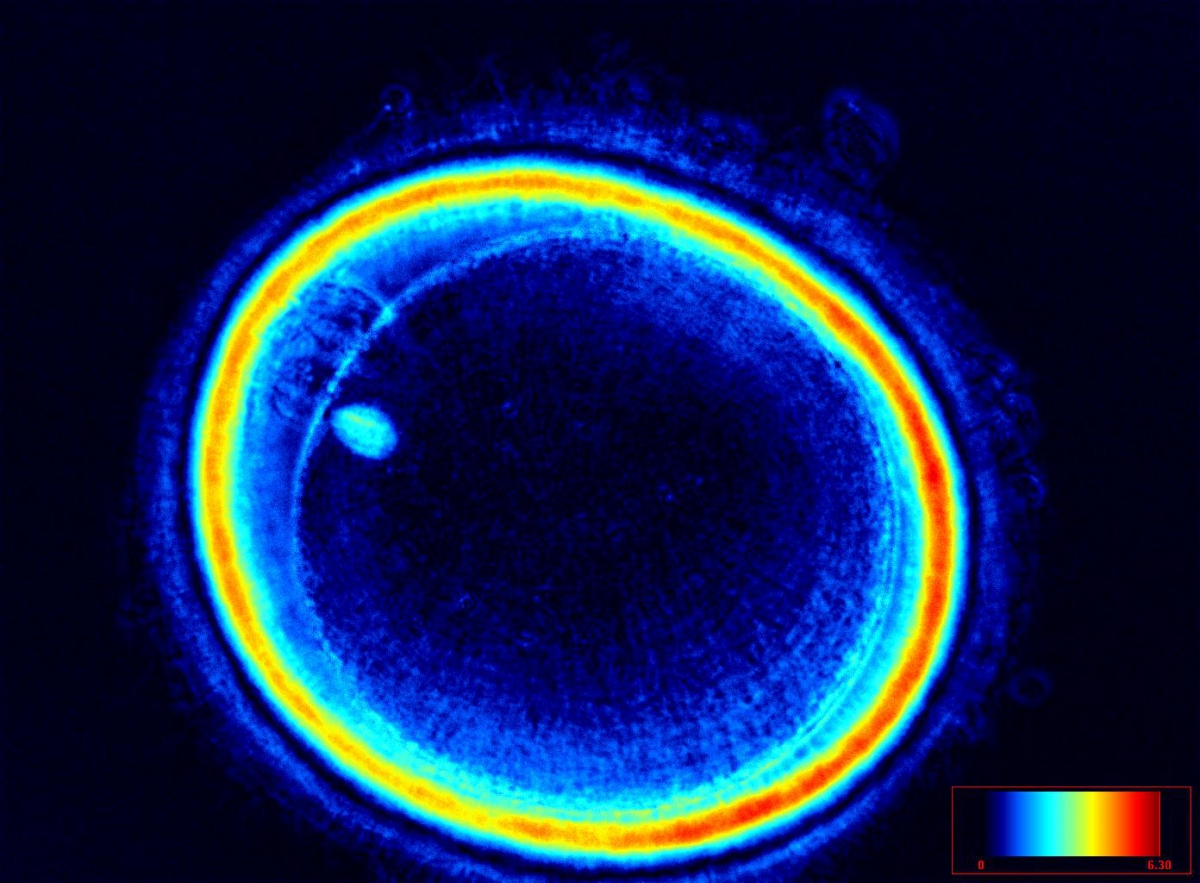
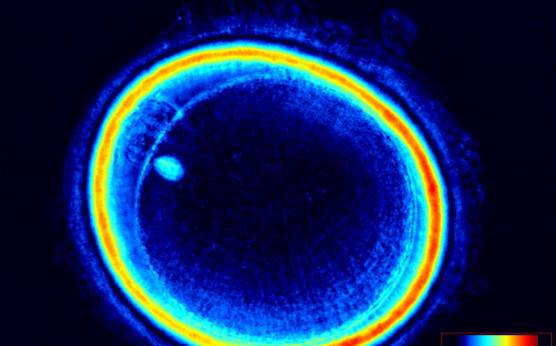
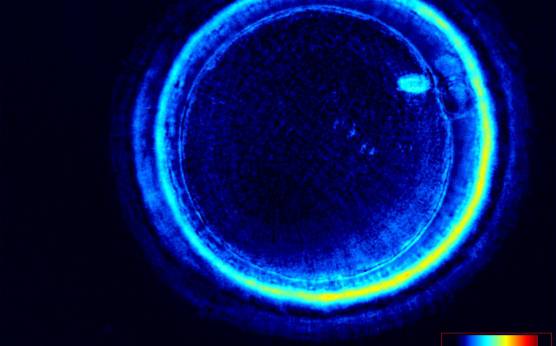
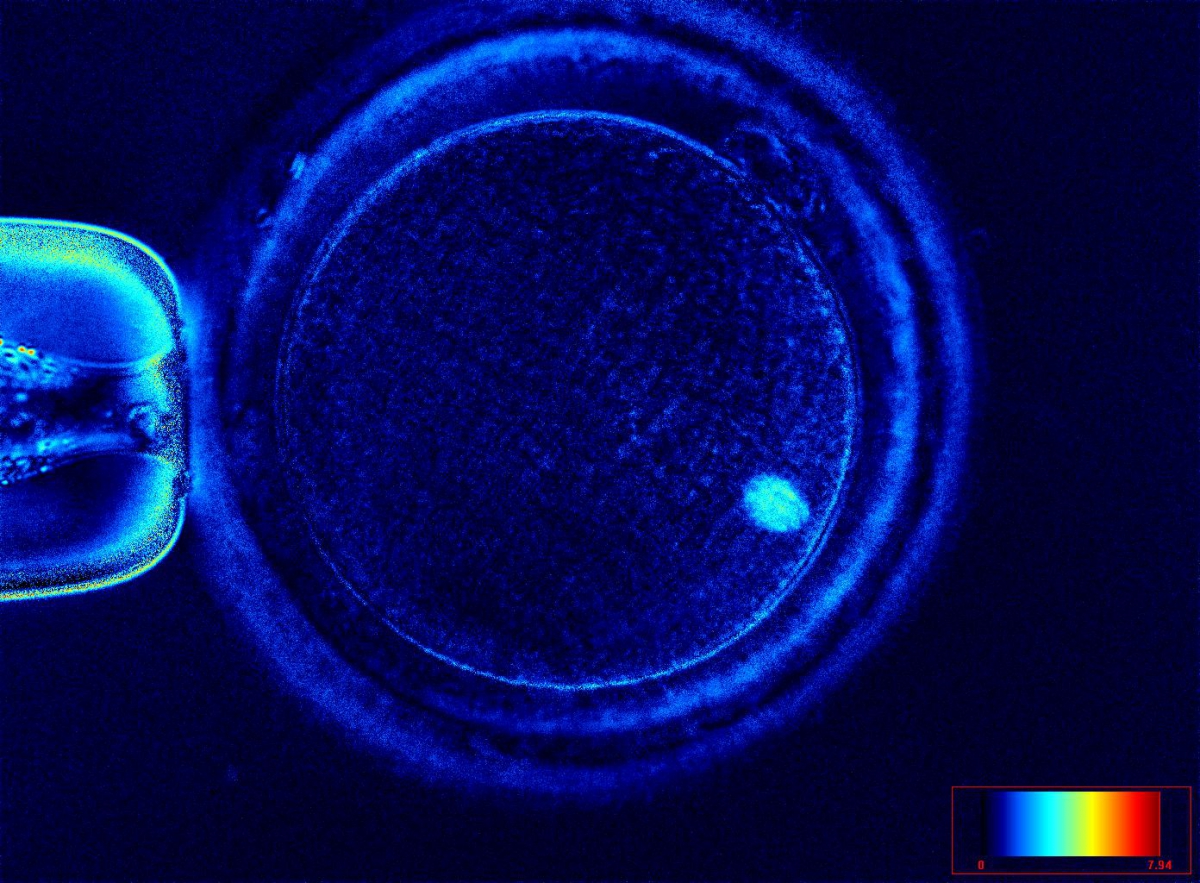
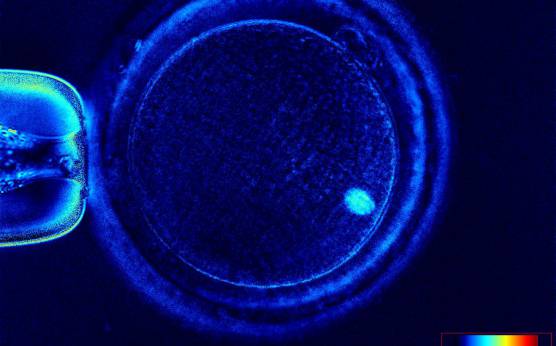
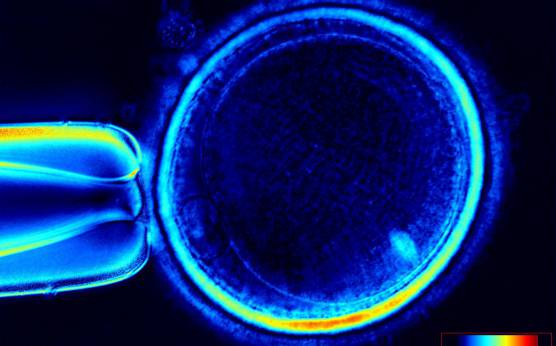
It has been shown that the presence of a detectable birefringent MS inside the oocyte cytoplasm of human MII oocytes (Fig. 52) might be an indicator of oocyte quality such as fertilization and developmental ability; however, retrospective analysis of results from published articles have been found to be controversial (Borini et al., 2005). A recent meta-analysis showed that although the visibility of the MS of MII oocytes is correlated with fertilization and embryo quality till the blastocyst stage of development, data were insufficient to demonstrate an impairment of the clinical pregnancy and implantation rates (Petersen et al., 2009). The main reason for the controversial findings was that some of the studies did not take into consideration the dynamics of spindle formation that are highly variable during the oocyte maturation stages as well as by confounding technical issues related to oocyte handling. It has been shown that spindle visualization changes during maturation, with a disappearance of the spindle for ∼1h expected during the maturation of MI to PBI extrusion at telophase I and then re-formation at the MII stage. The microtubules of the MS are highly sensitive to chemical (hyaluronidase) and physical changes (temperature and pH variations) that may occur during oocyte handling and a reversible disappearance of the spindle can be expected during these technical preparations (Rienzi et al., 2003; De Santis et al., 2005; Montag et al., 2006).
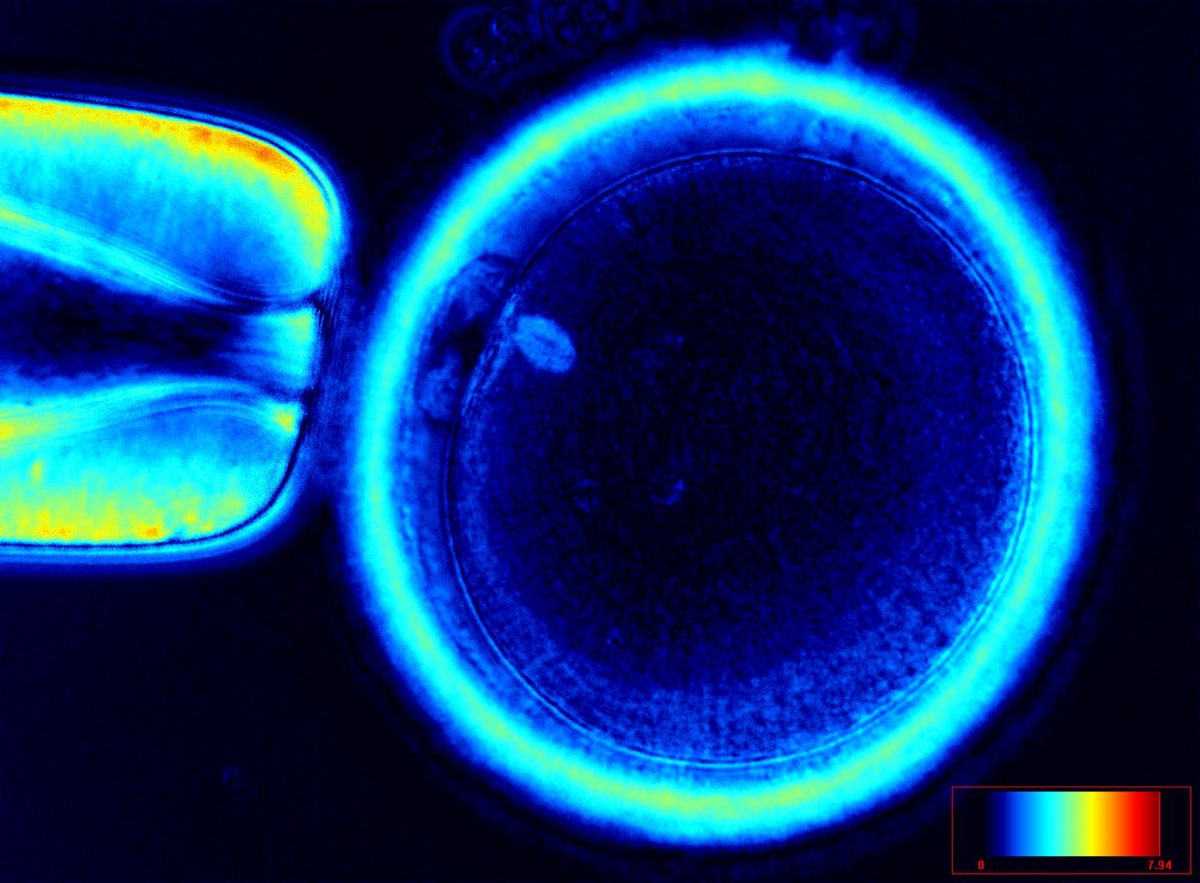
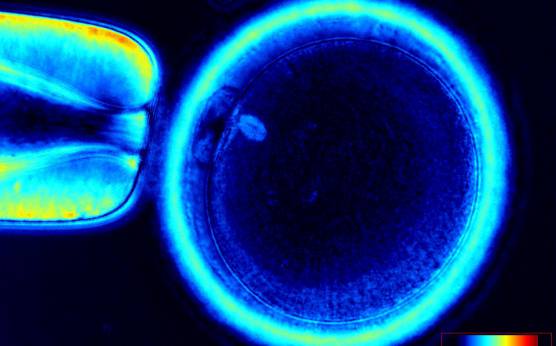
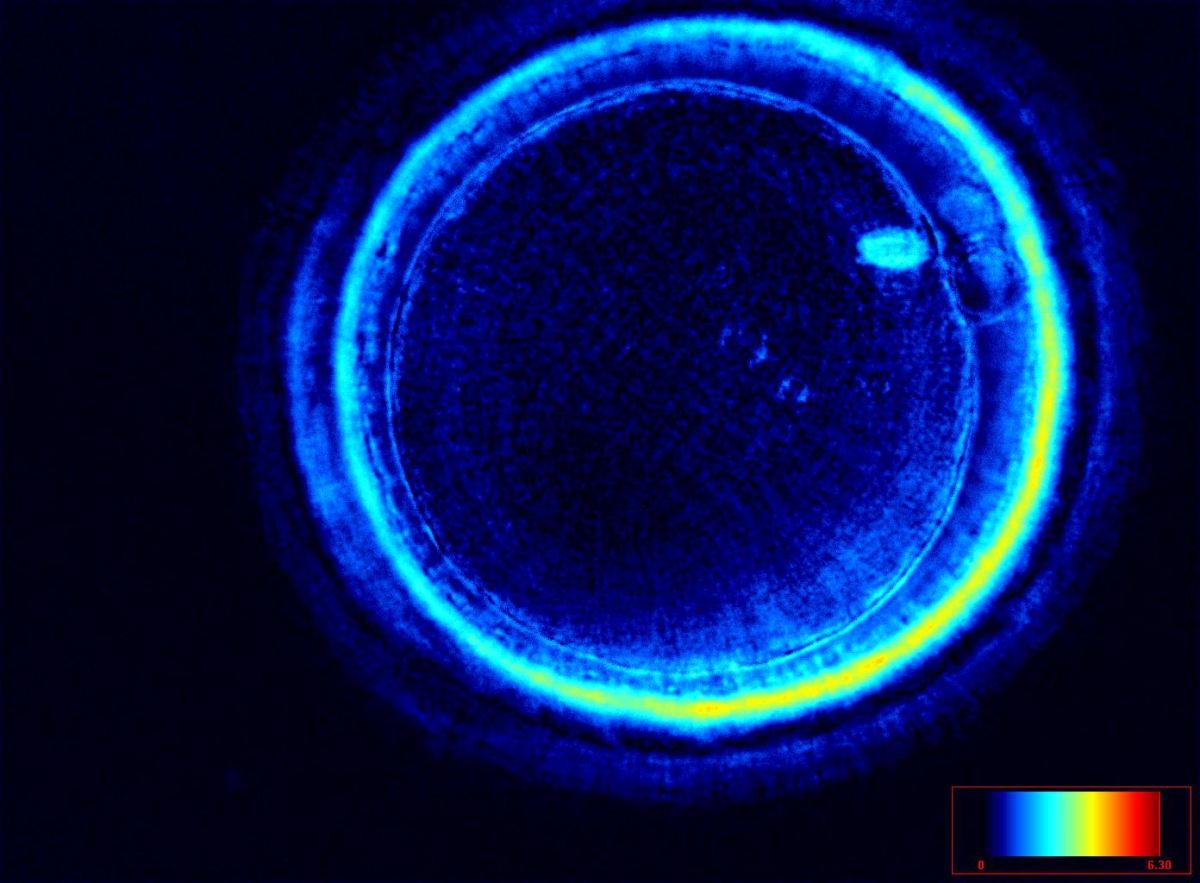
The spindle structure, when detectable, is not always aligned with the PBI in MII oocytes, and the relationship between the degree of MS deviation from the location of the PBI may also play a role in clinical outcome (Rienzi et al., 2003, 2005; Figs 53–58). Although it has been shown that there is no relationship between the displacement of the PBI with regard to the MS position on fertilization outcomes and embryo quality (Rienzi et al., 2003; Rama Raju et al., 2007), fertilization is impaired when the displacement is >90% (Rienzi et al., 2003; Figs 59 and 60). Slight shifts in the position of the PBI may also be related to physical displacement during the denudation processes, and can be reversible. However, when the displacement is so great because of inappropriate handling, it could cause irreversible damage to the oocyte structure.
Therefore, a precise sequential analysis of MS imaging should be performed after possible chemical and physical changes and immediately prior to ICSI if MS characteristics are to be used as possible markers of oocyte quality and viability.
Article references:
Alpha Scientists in Reproductive medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 2011;26:1270-1283.
Abstract/FREE Full Text
Balaban B, Urman B. Effect of oocyte morphology on embryo development and implantation. Reprod Biomed Online 2006;12:608-615.
Medline | Web of Science | Google Scholar
Borini A, Lagalla C, Cattoli M, Sereni E, Sciajno R, Flamigni C, Coticchio G. Predictive factors for embryo implantation potential. Reprod Biomed Online 2005;10:653-668.
Medline | Web of Science | Google Scholar
De Santis L, Cino I, Rabellotti R, Calzi F, Persico P, Borini A, Coticchio G. Polar body morphology and spindle imaging as predictors of oocyte quality. Reprod BioMed Online 2005;11:36-42.
CrossRef | Medline | Web of Science | Google Scholar
Ebner T, Moser M, Tews G. Is oocyte morphology prognostic of embryo developmental potential after ICSI? Reprod Biomed Online 2006;12:507-512.
Medline | Web of Science | Google Scholar
Montag M, Schimming T, Van der Ven H. Spindle imaging in human oocytes: the impact of the meiotic cycle. Reprod Biomed Online 2006;12:442-446.
Medline | Web of Science | Google Scholar
Otsuki J, Nagai Y, Chiba K. Lipofuscin bodies in human oocytes as an indicator of oocyte quality. J Assist Reprod Genet 2007;24:263-270.
CrossRef | Medline | Web of Science | Google Scholar
Rama Raju GA, Prakash GJ, Krishna KM, Madan K. Meiotic spindle and zona pellucida characteristics as predictors of embryonic development: a preliminary study using PolScope imaging. Reprod Biomed Online 2007;14:166-174.
CrossRef | Medline | Web of Science | Google Scholar
Rienzi L, Ubaldi F, Martinez F, Iacobelli M, Minasi MG, Ferrero S, Tesarik J, Greco E. Relationship between meiotic spindle location with regard to the polar body position and oocyte developmental potential after ICSI. Hum Reprod 2003;18:1289-1293.
Abstract/FREE Full Text
Rienzi L, Ubaldi FM, Iacobelli M, Minasi MG, Romano S, Greco E. Meiotic spindle visualization in living human oocytes. Reprod Biomed Online 2005;10:192-198.
CrossRef | Medline | Web of Science | Google Scholar