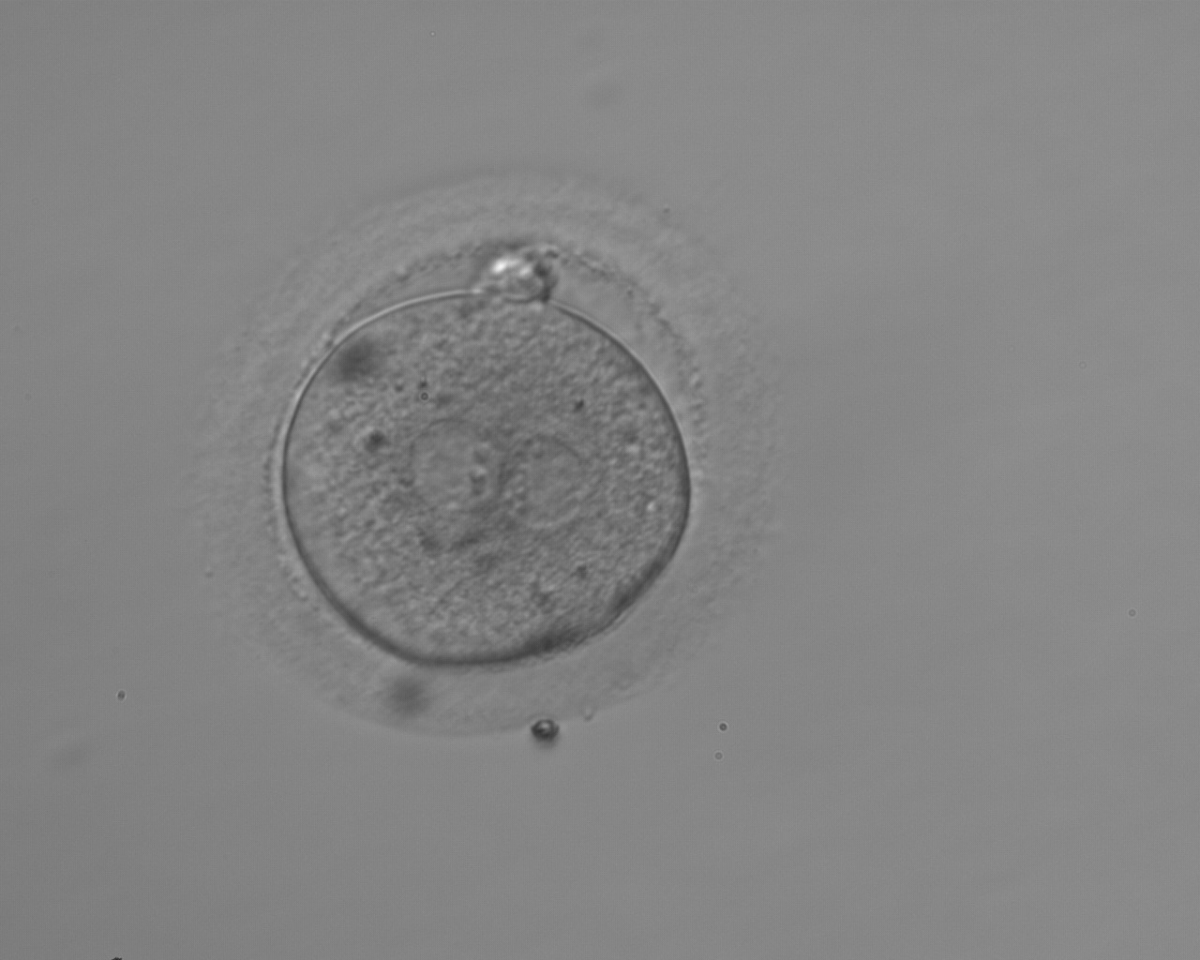
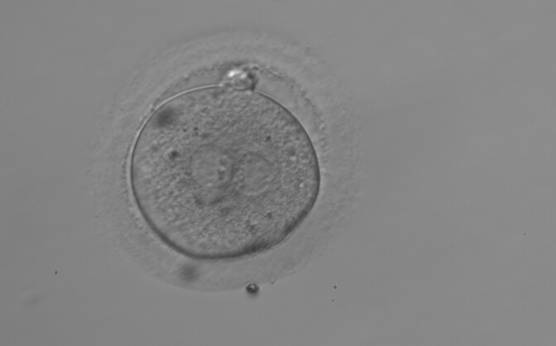
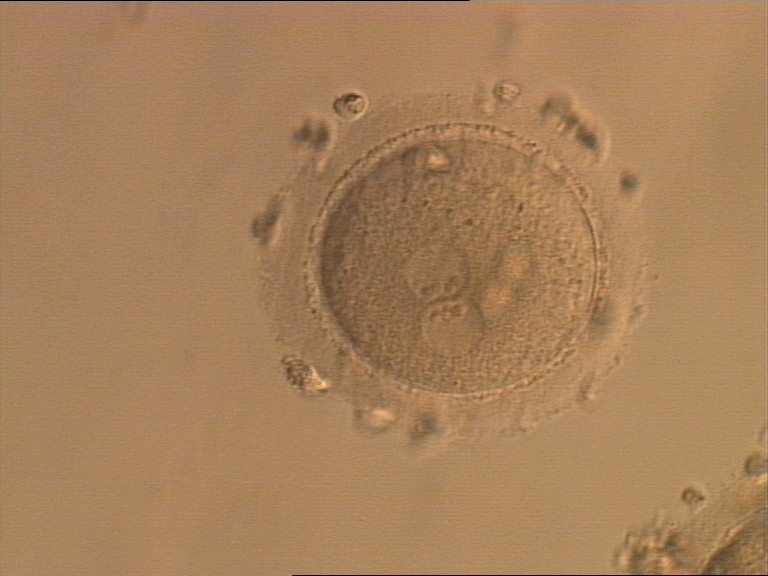
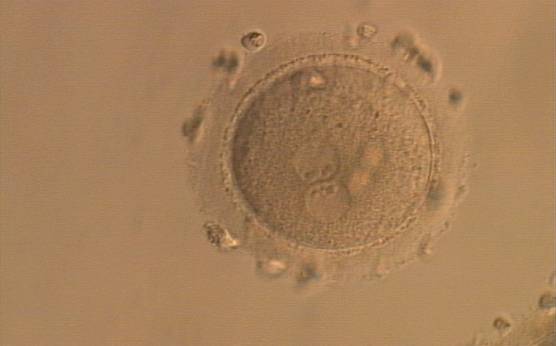
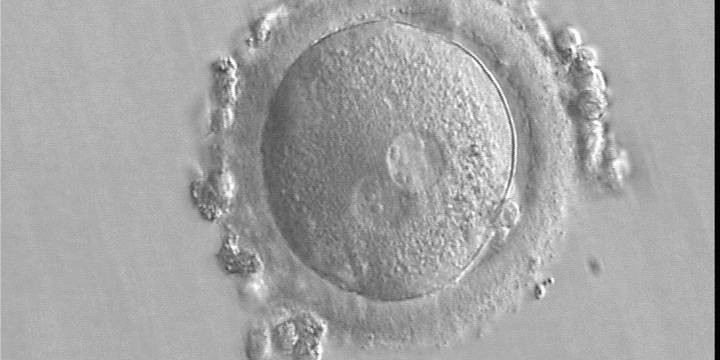
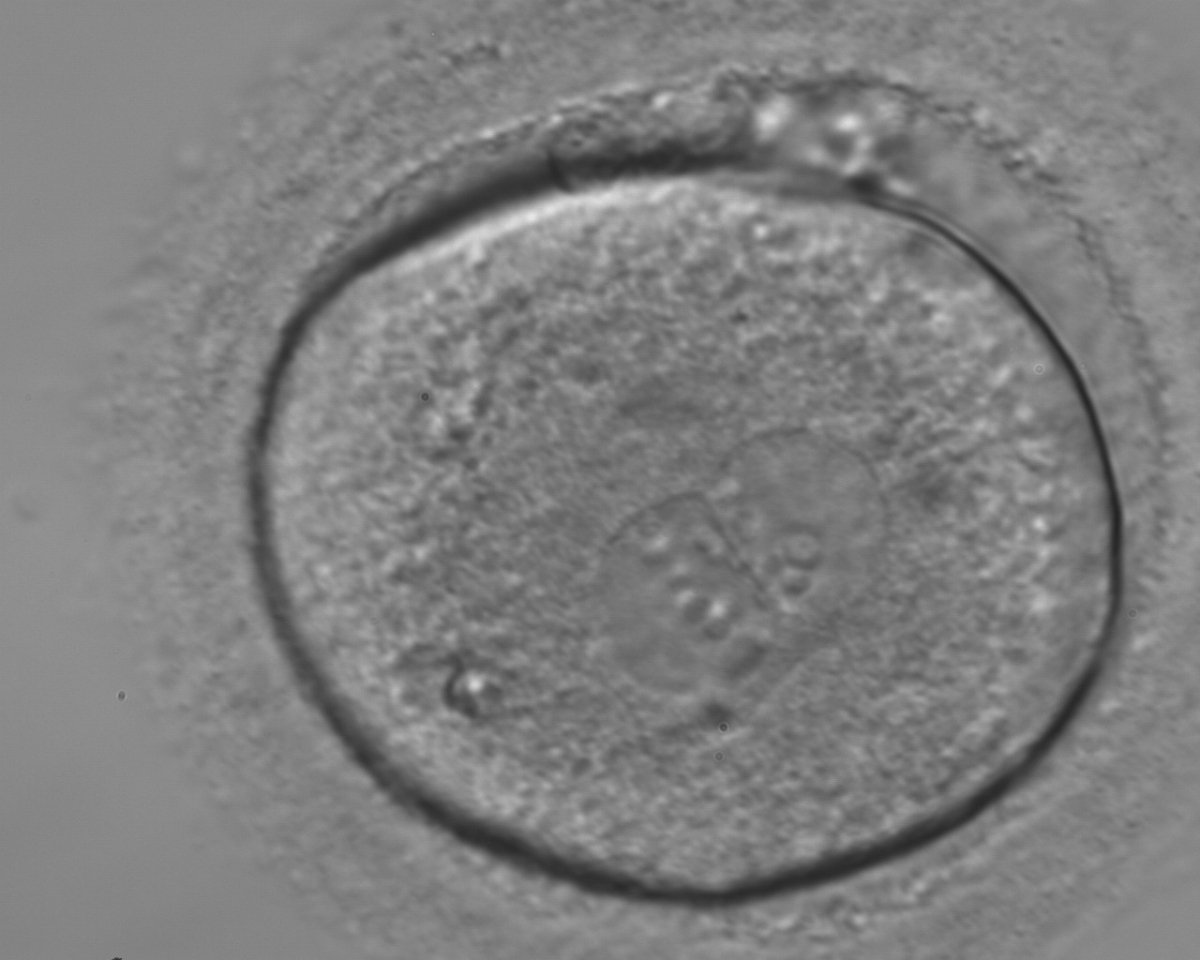
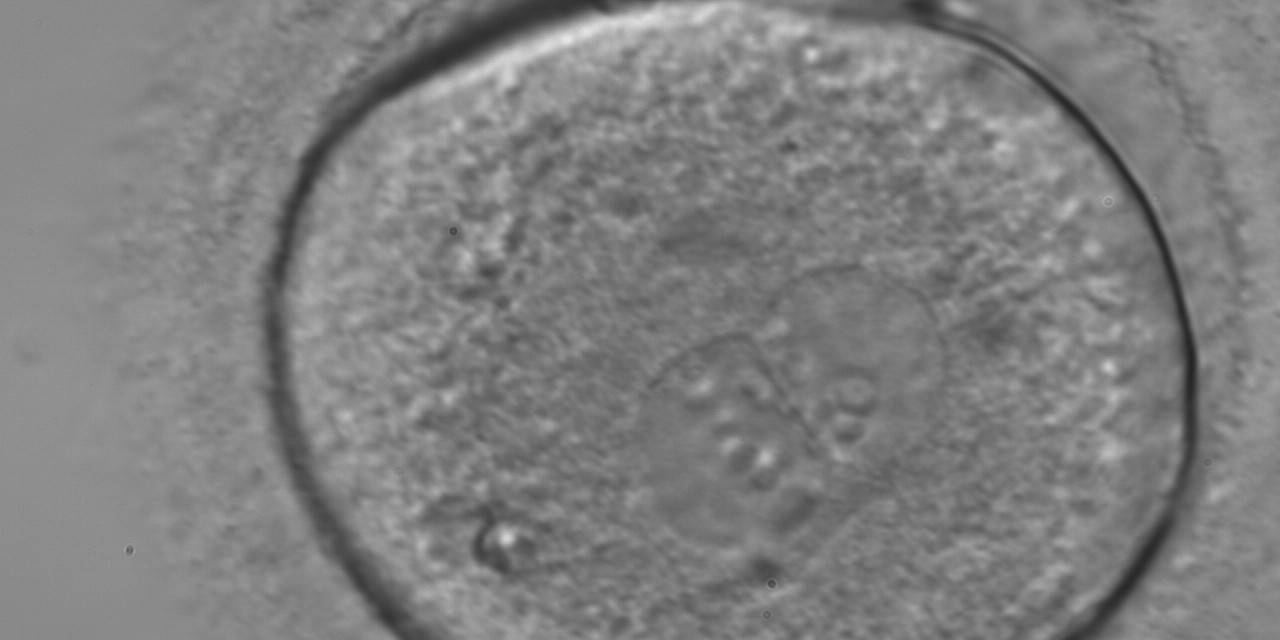
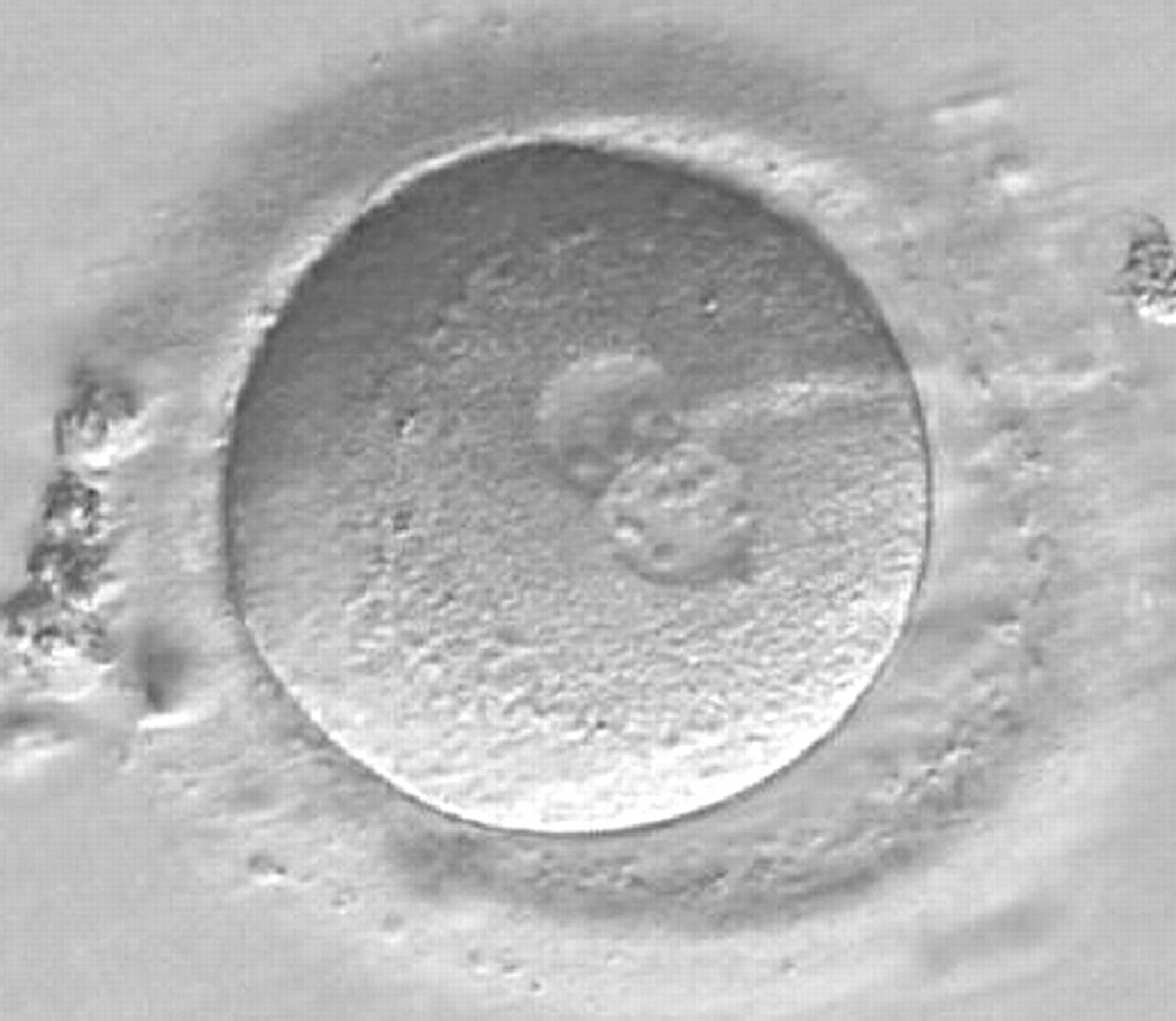
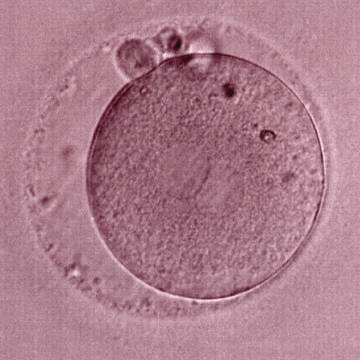
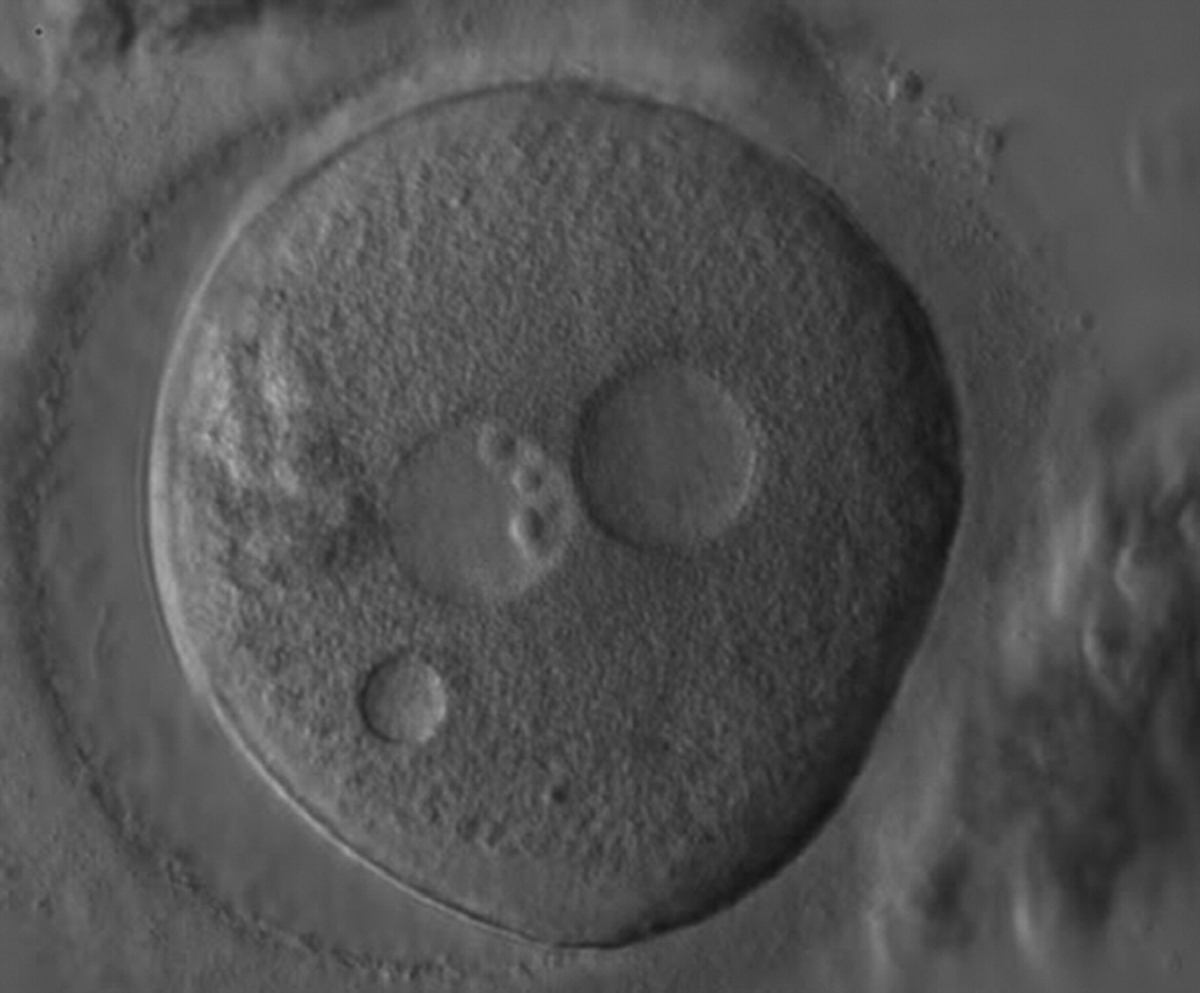
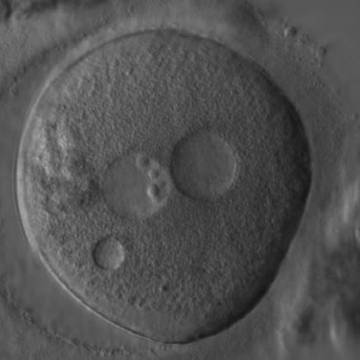
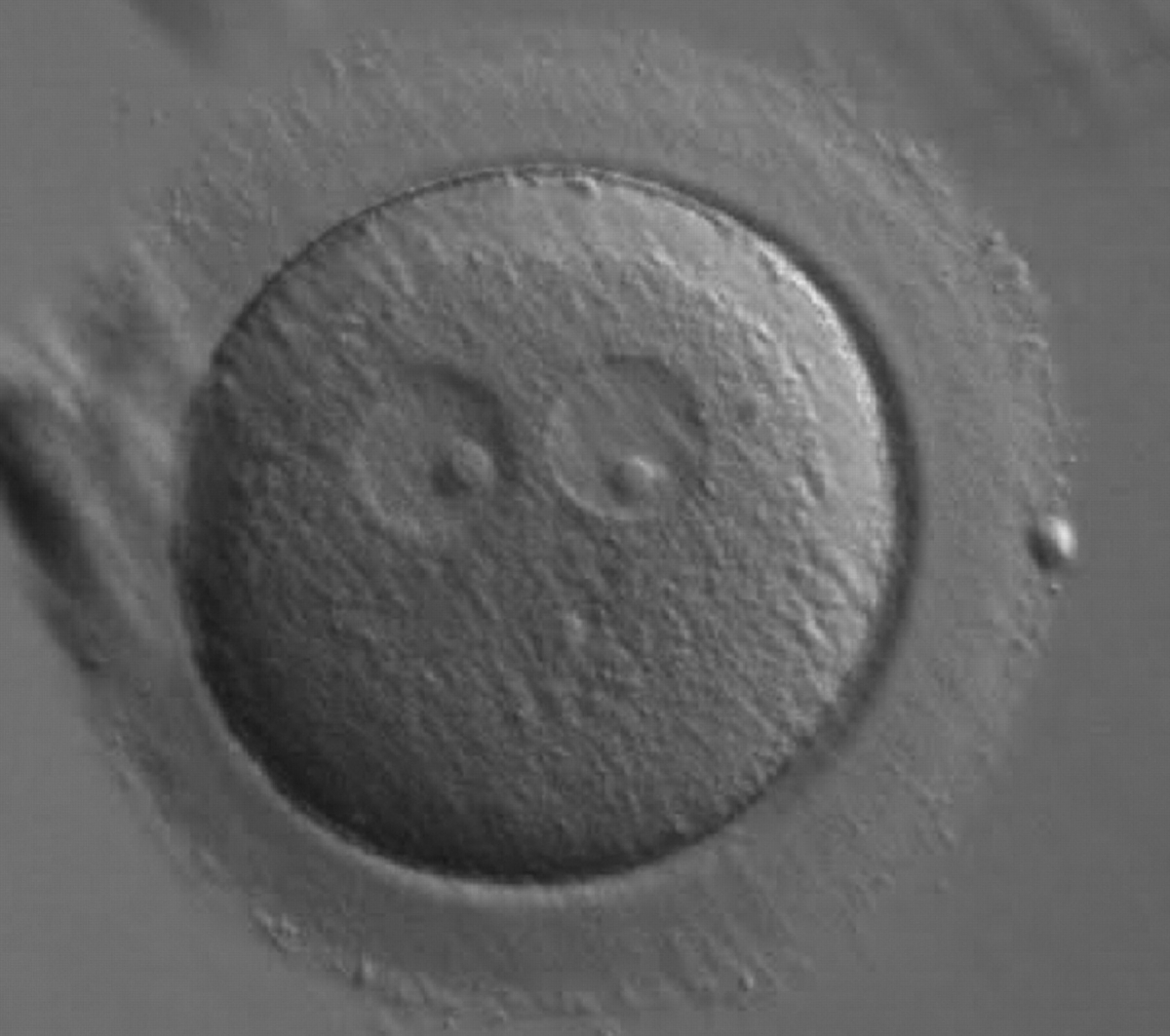
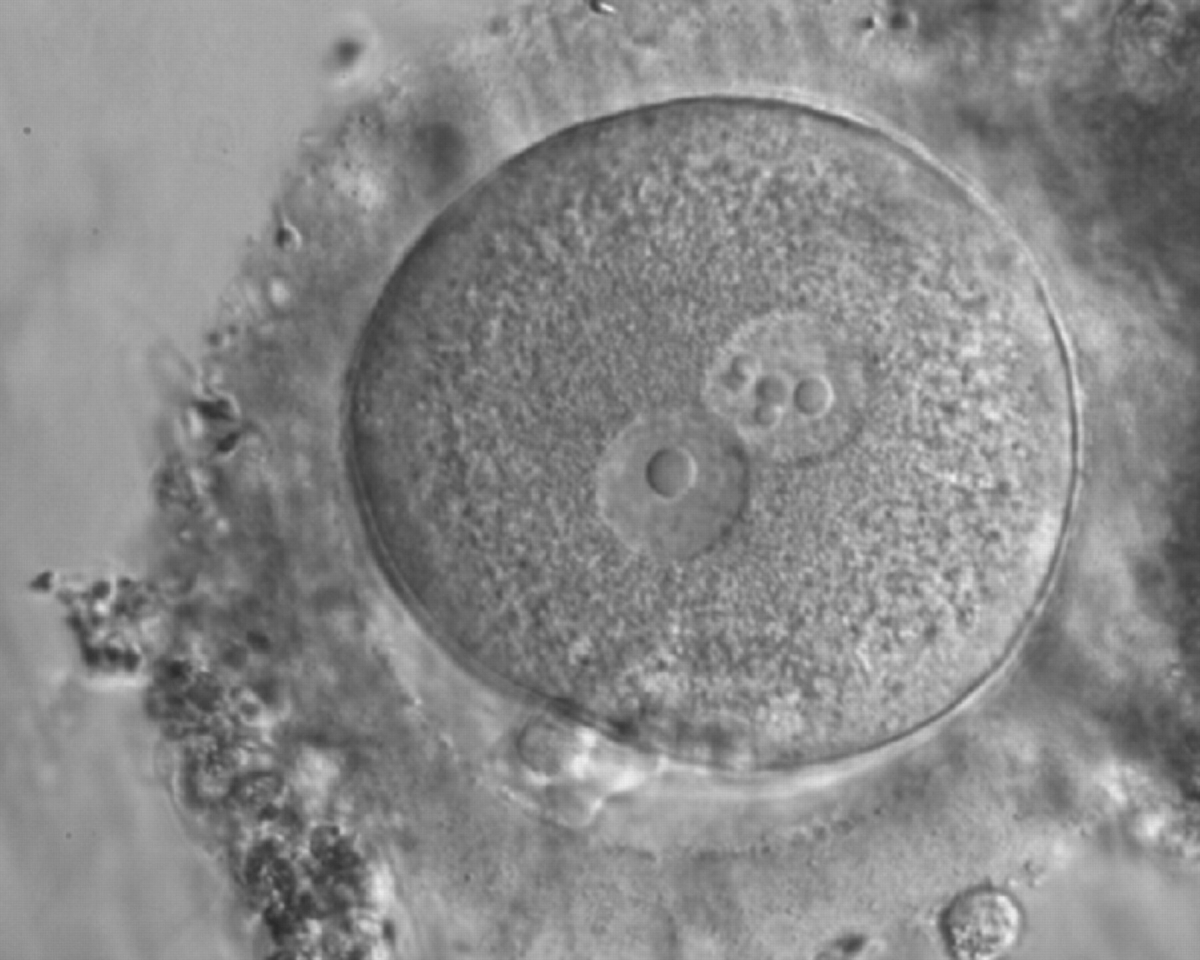
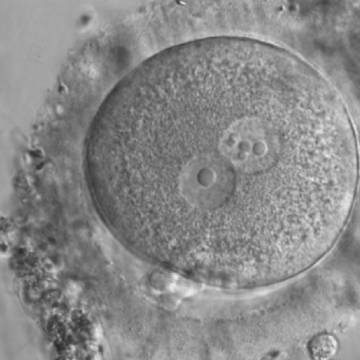
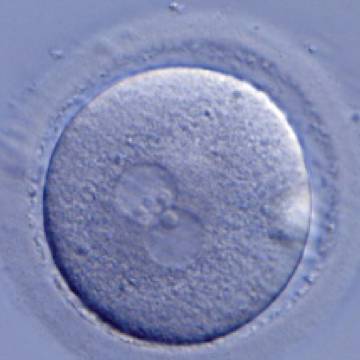
D. Nucleolar precursor bodies
D.1 NPBs: aligned/scattered/differential alignment between PNs
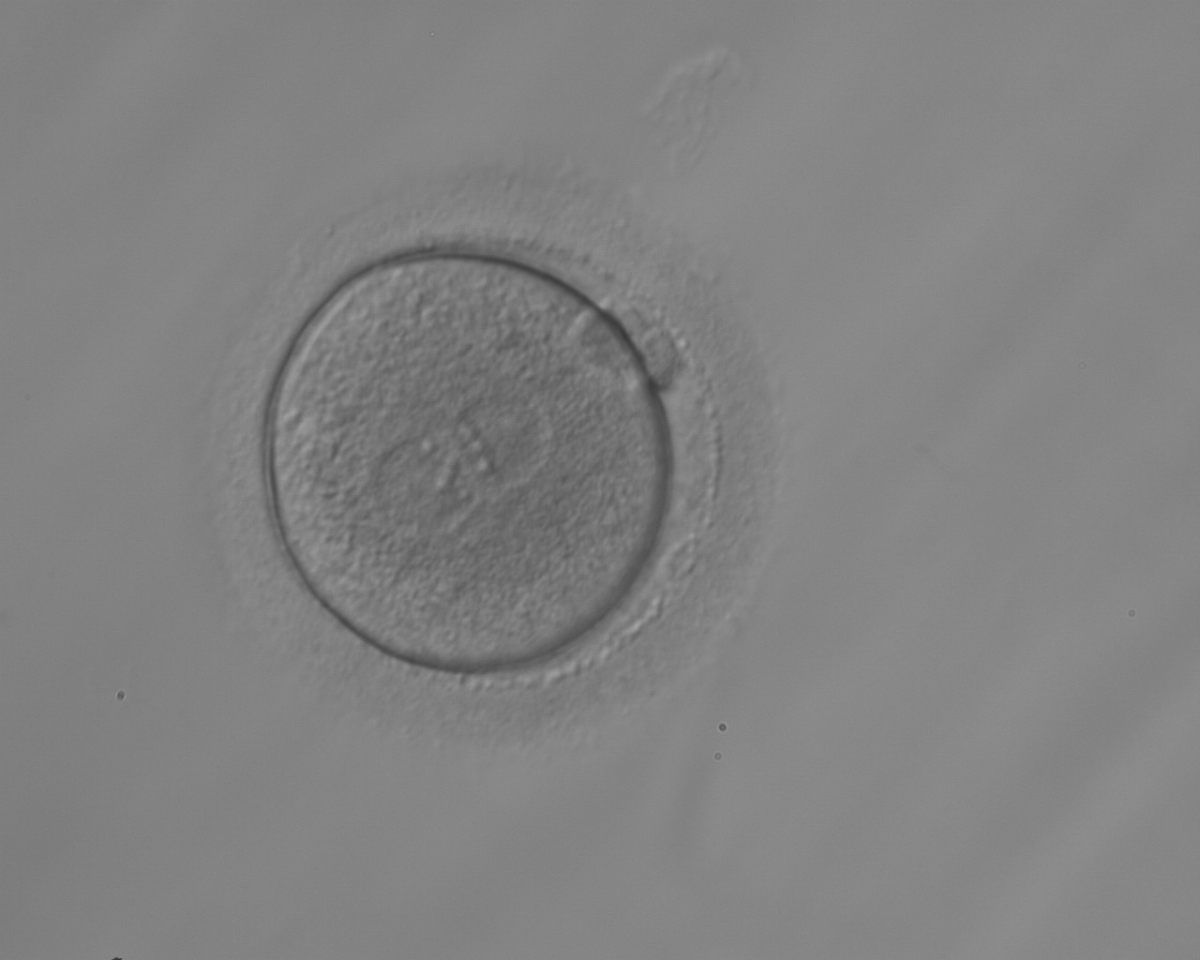
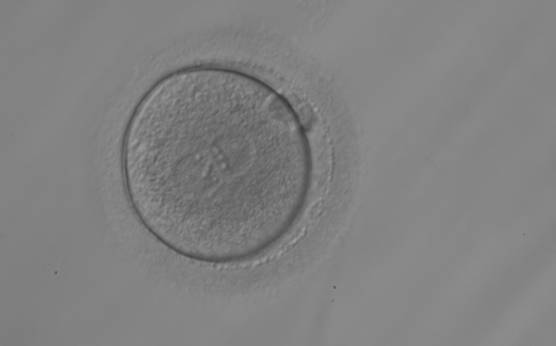
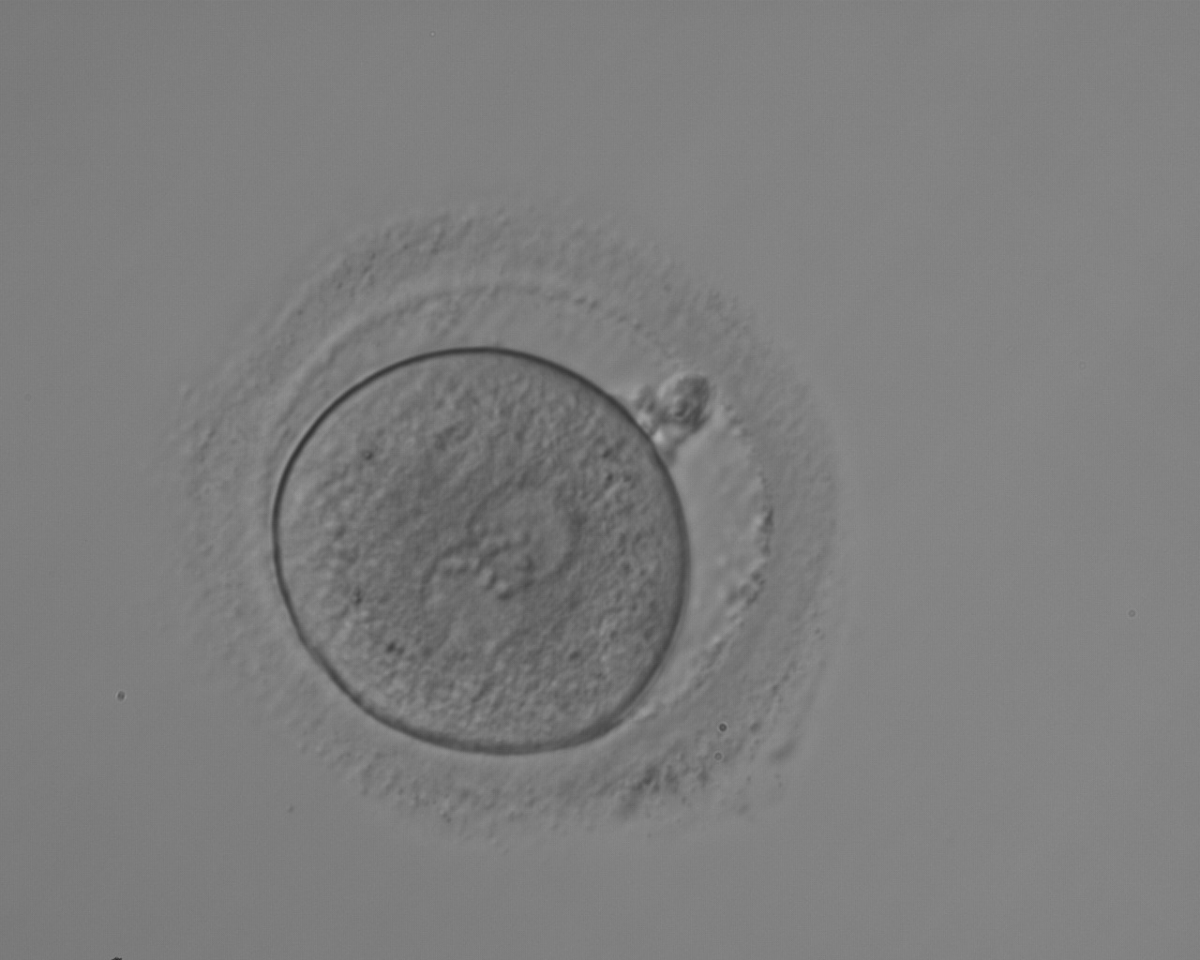
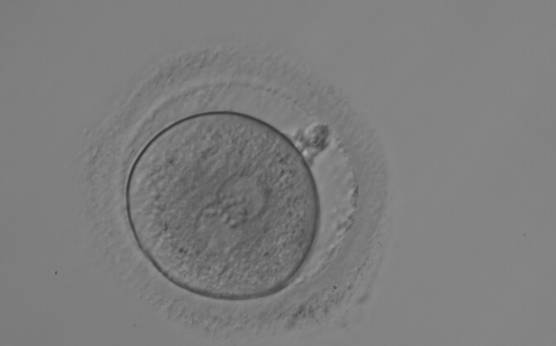
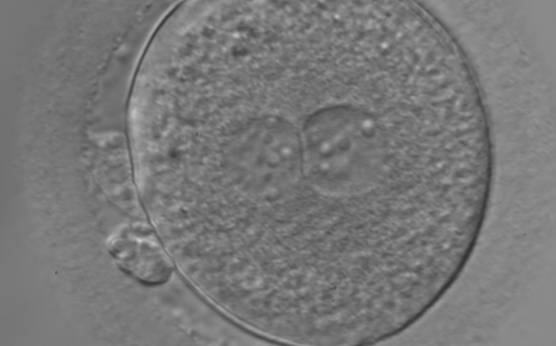
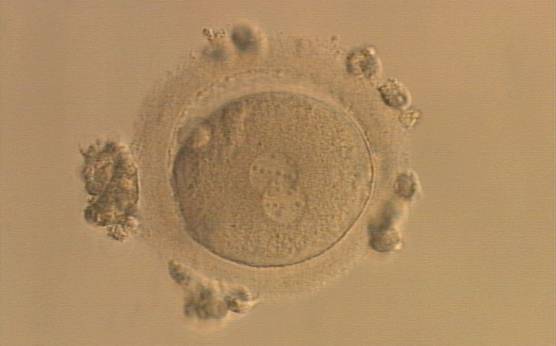
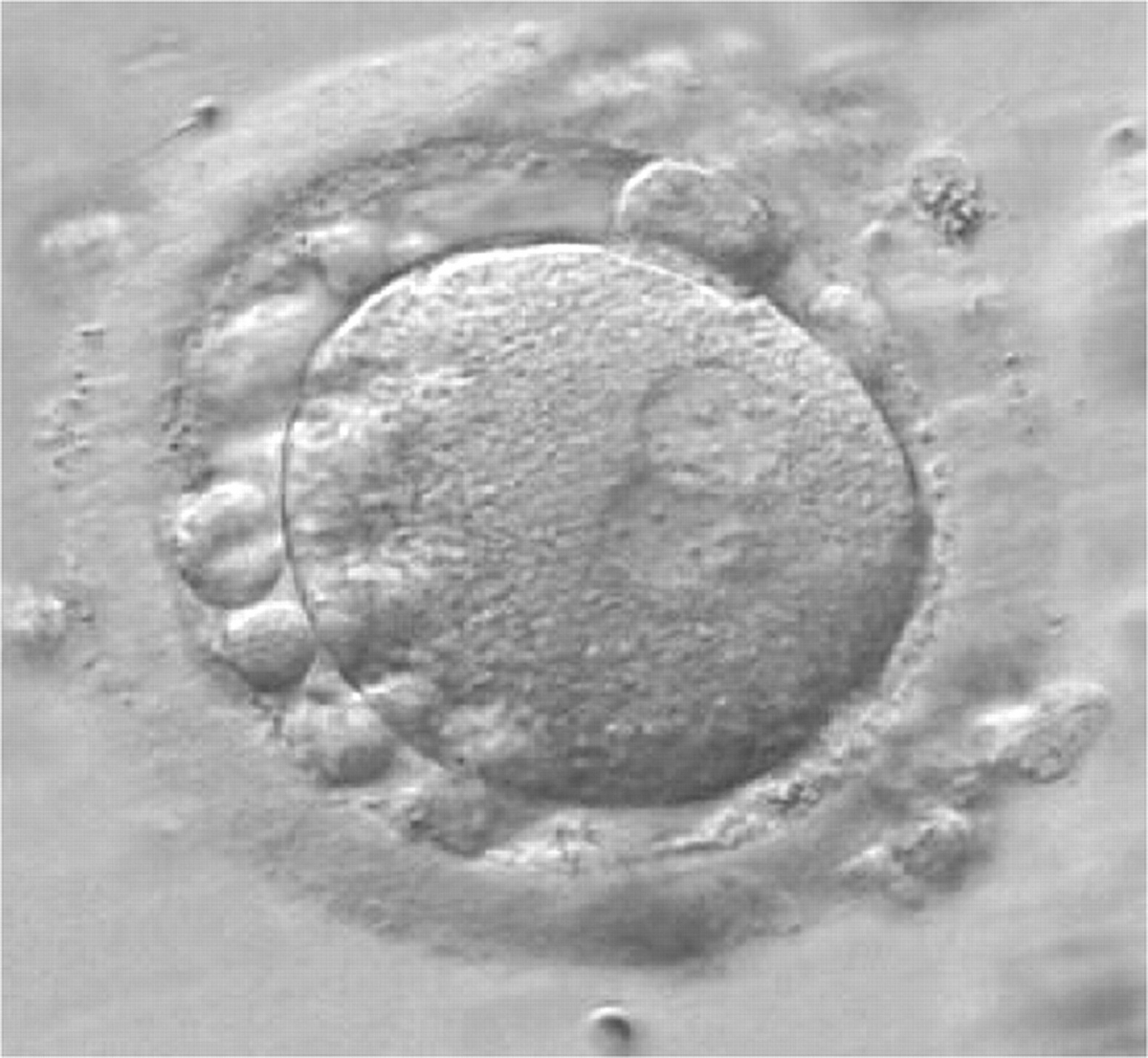
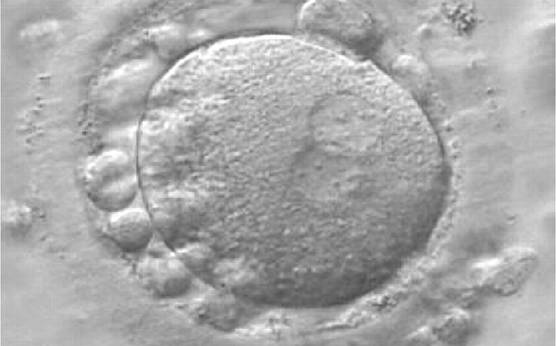
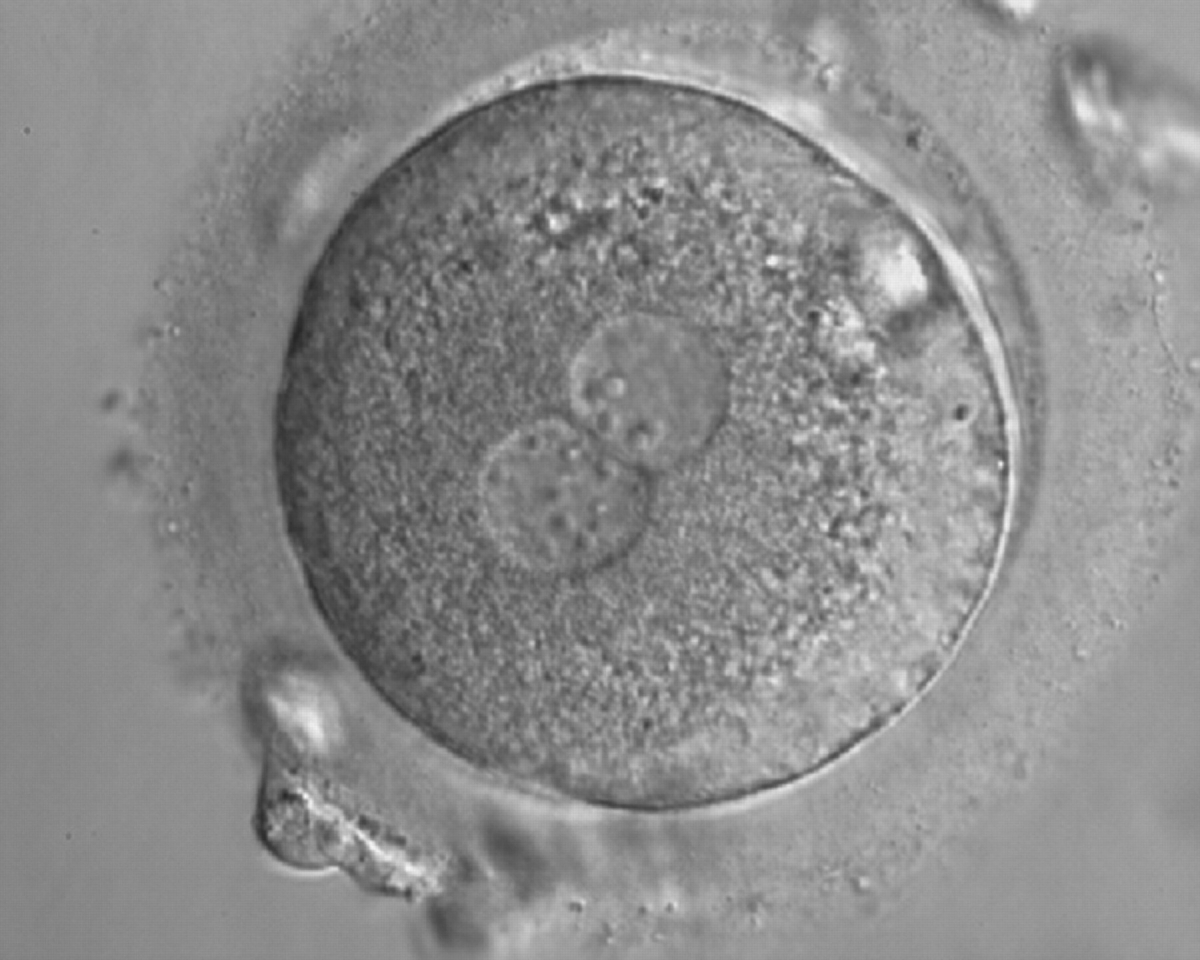
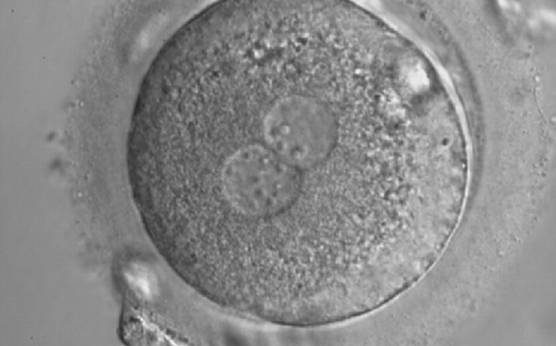
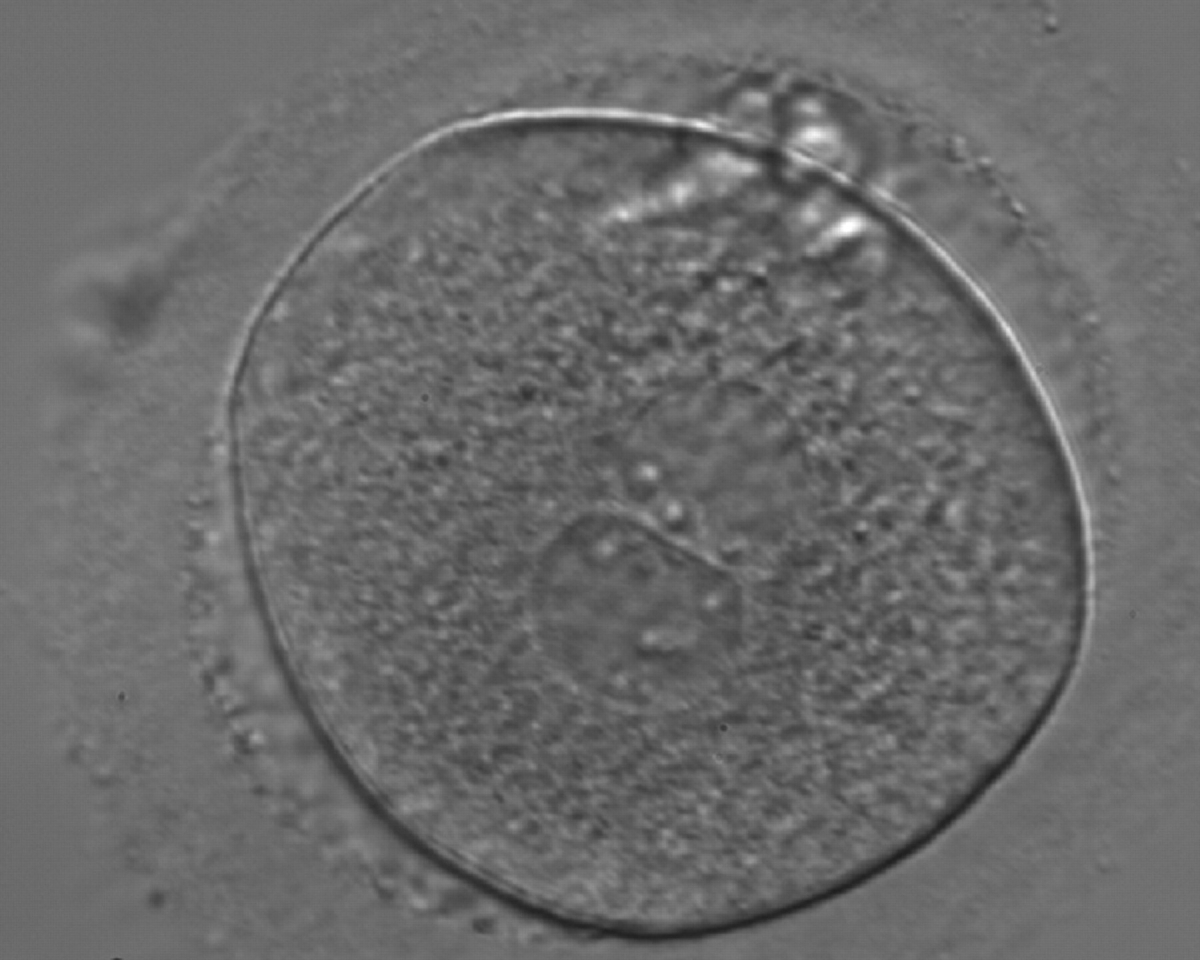
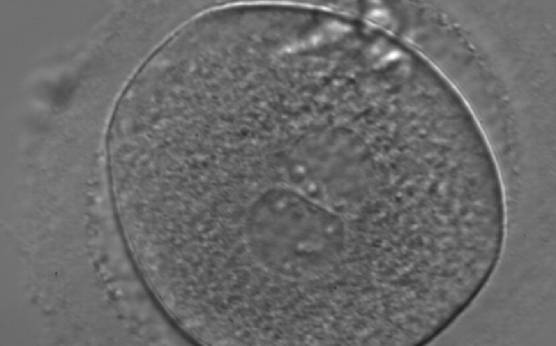
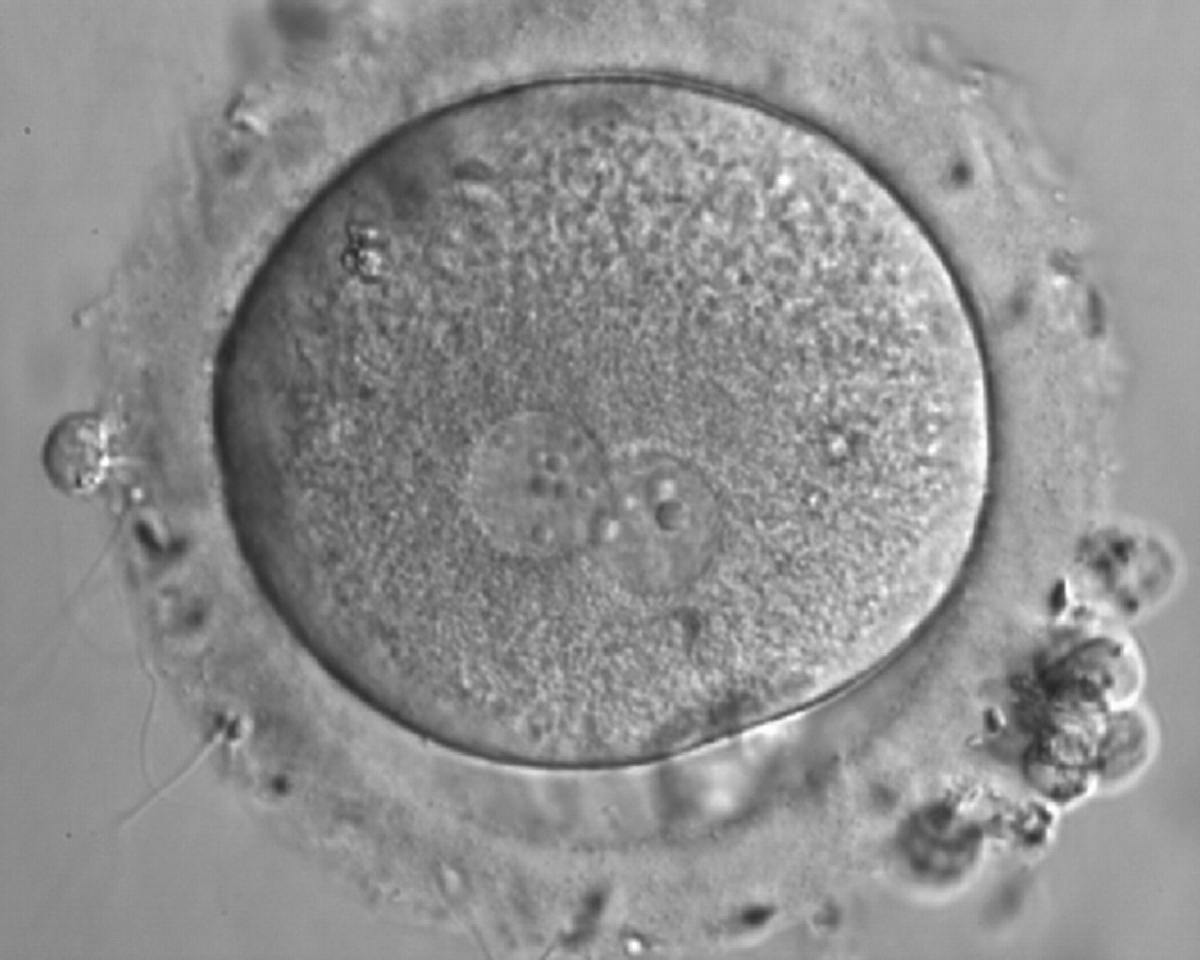
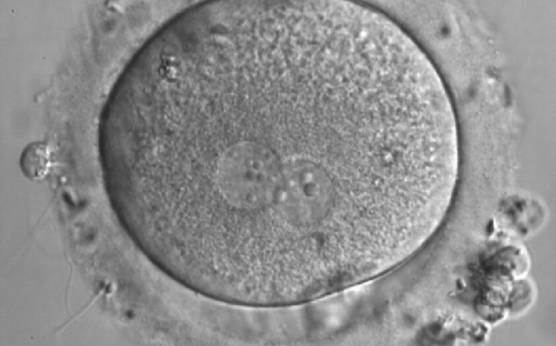
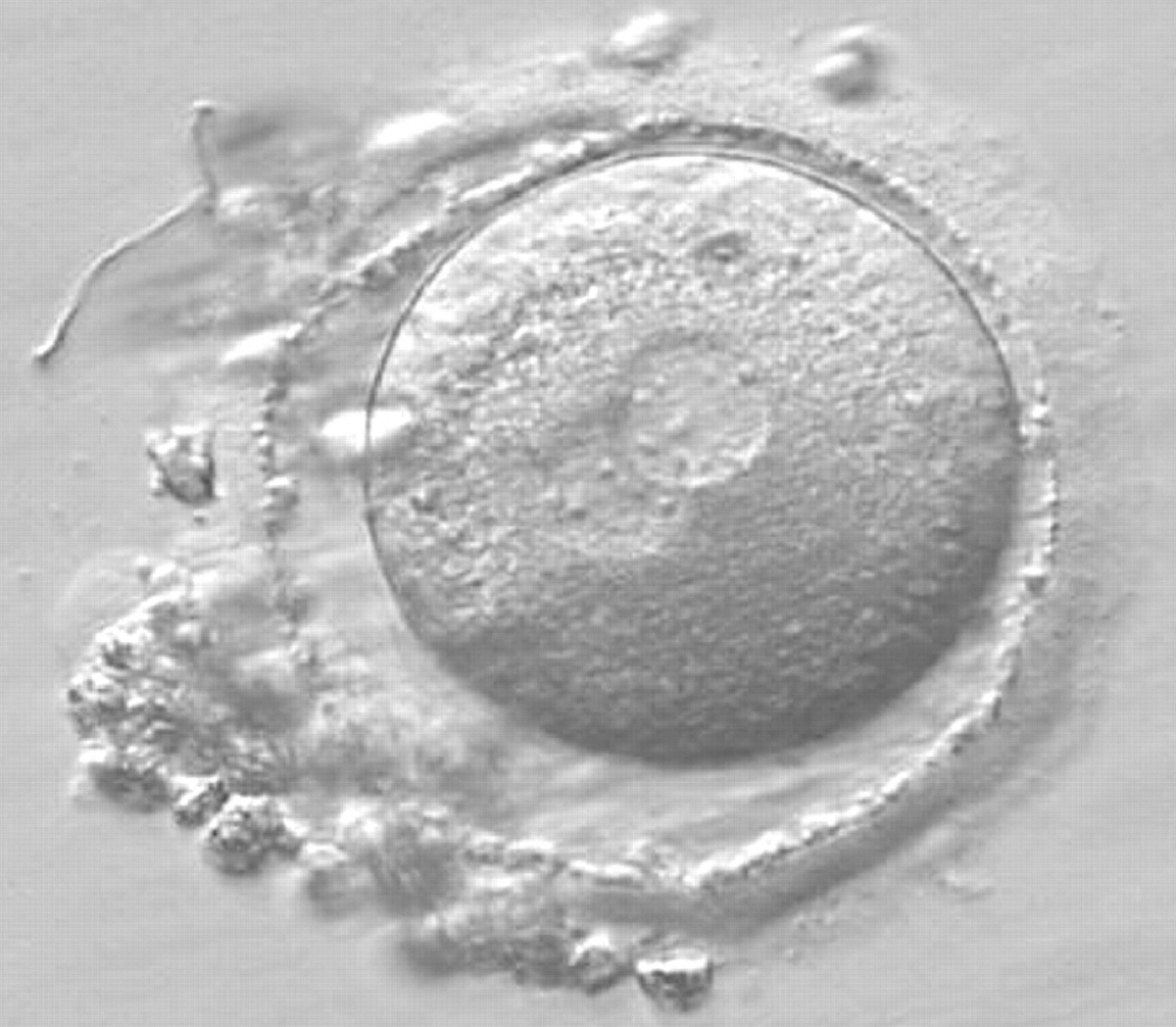
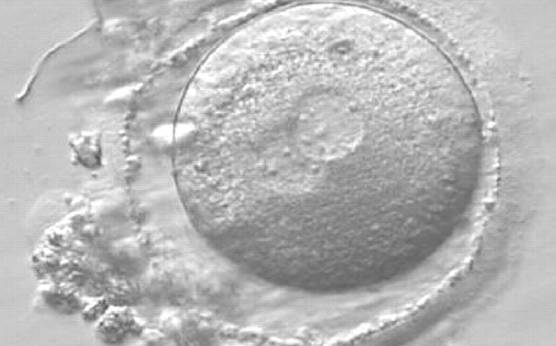
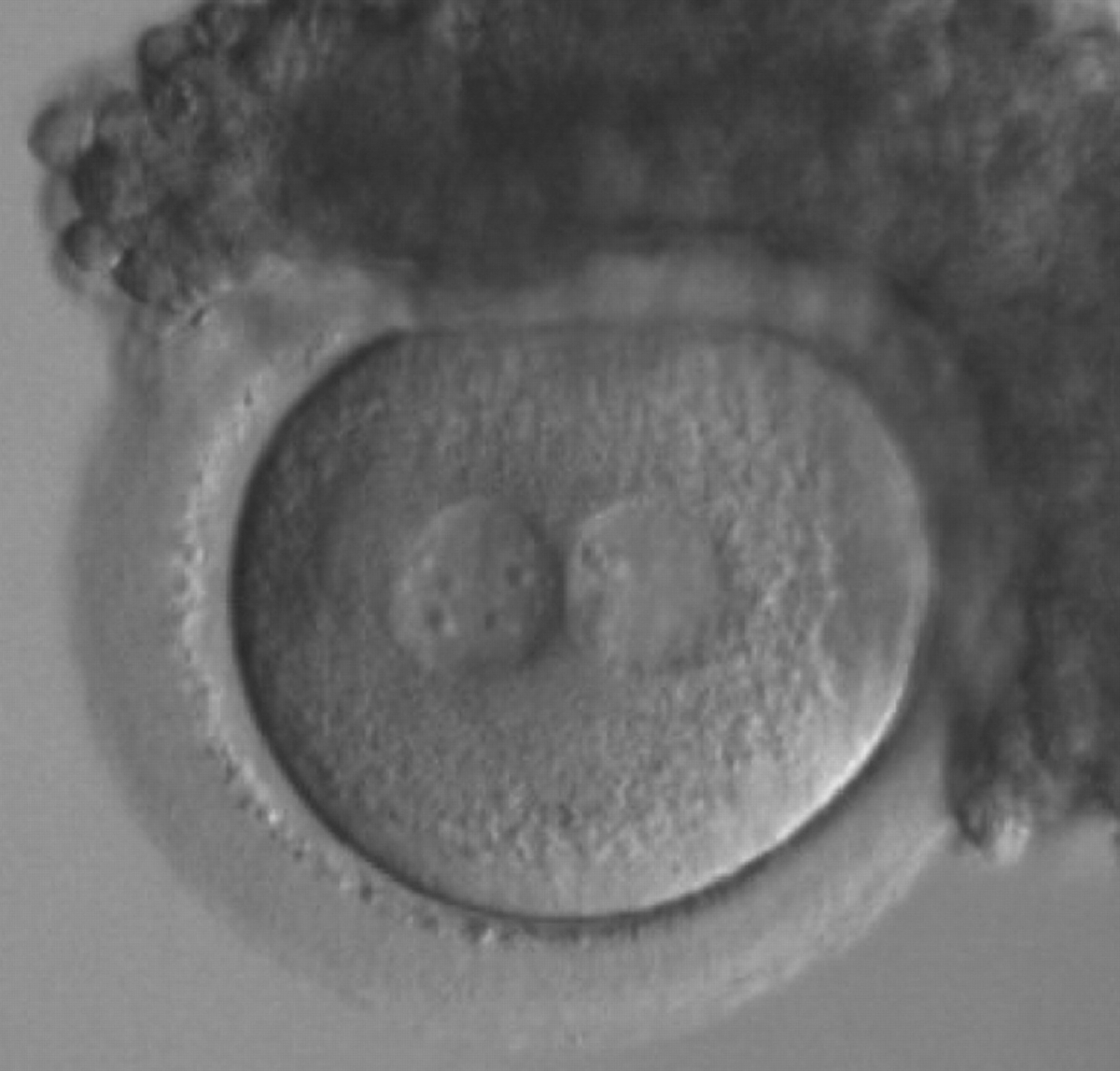
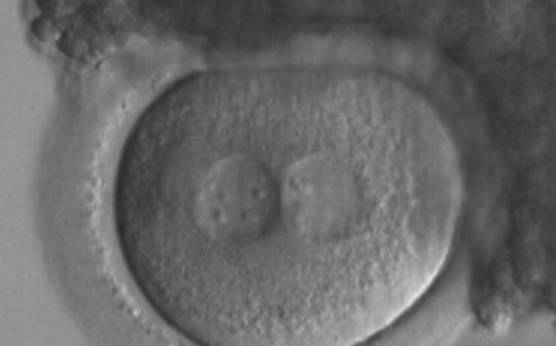
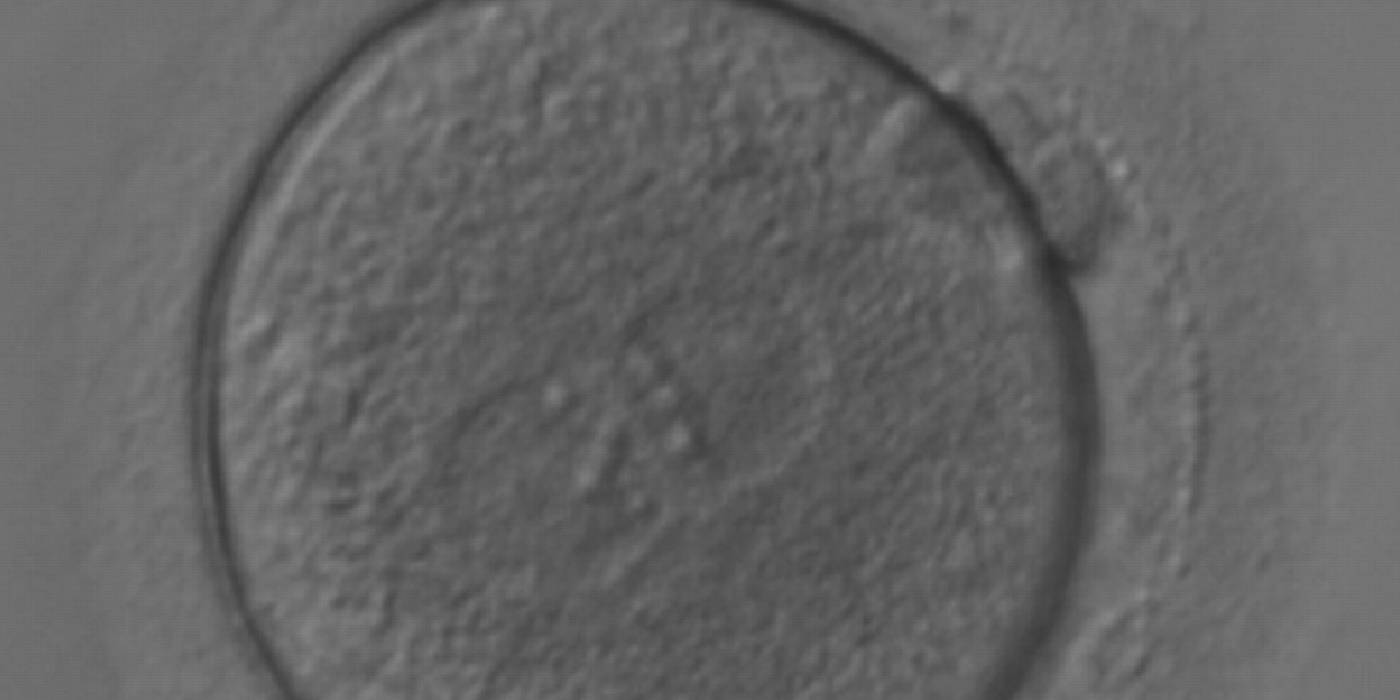
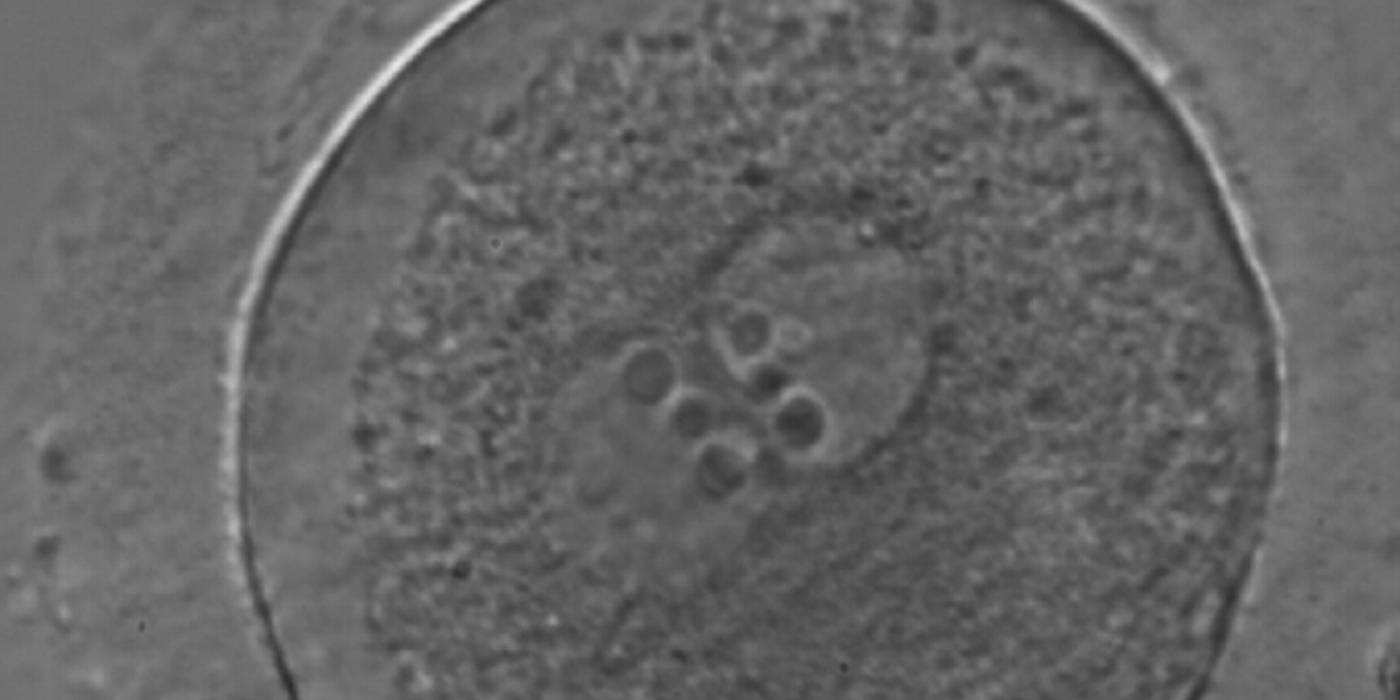
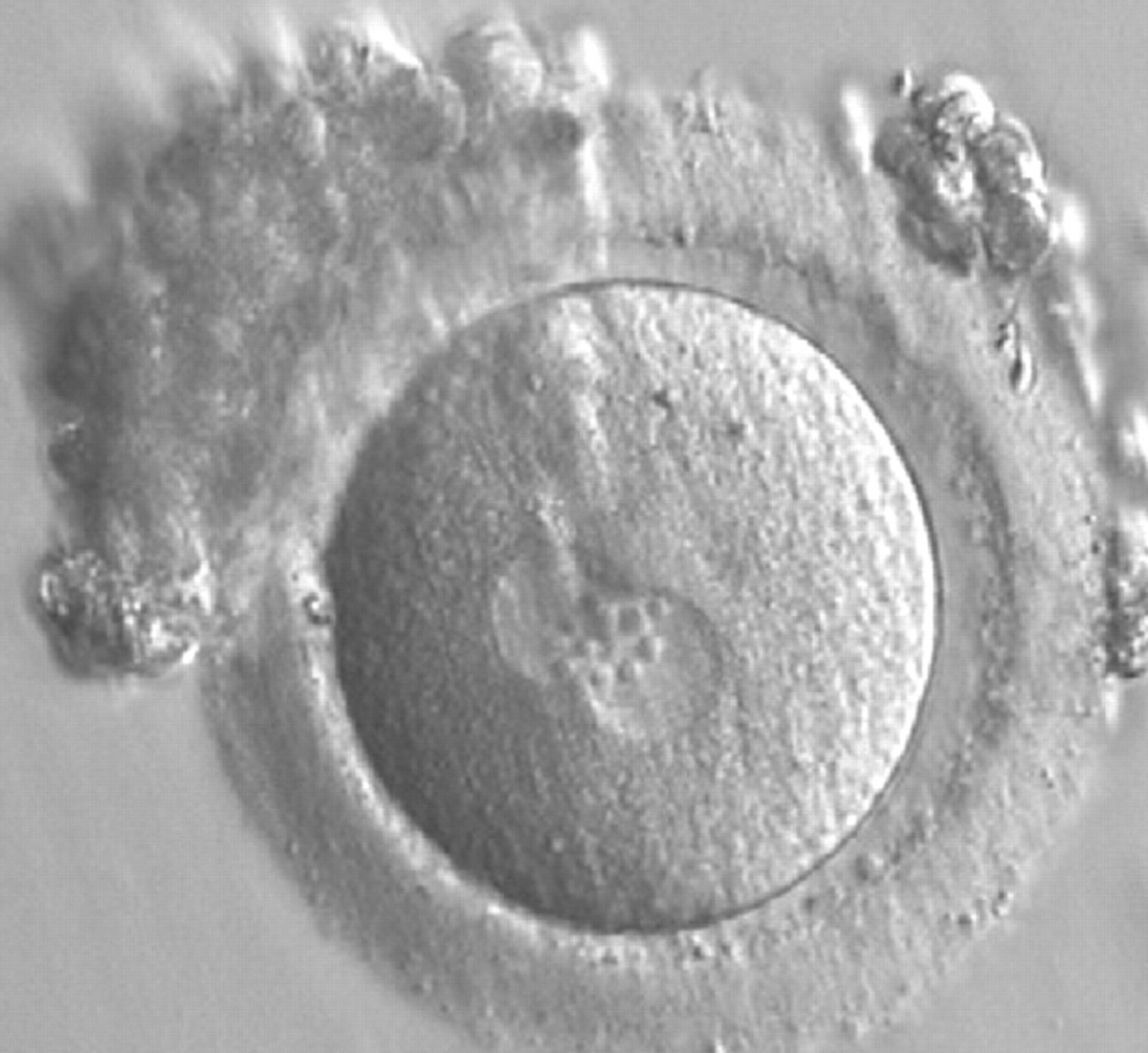
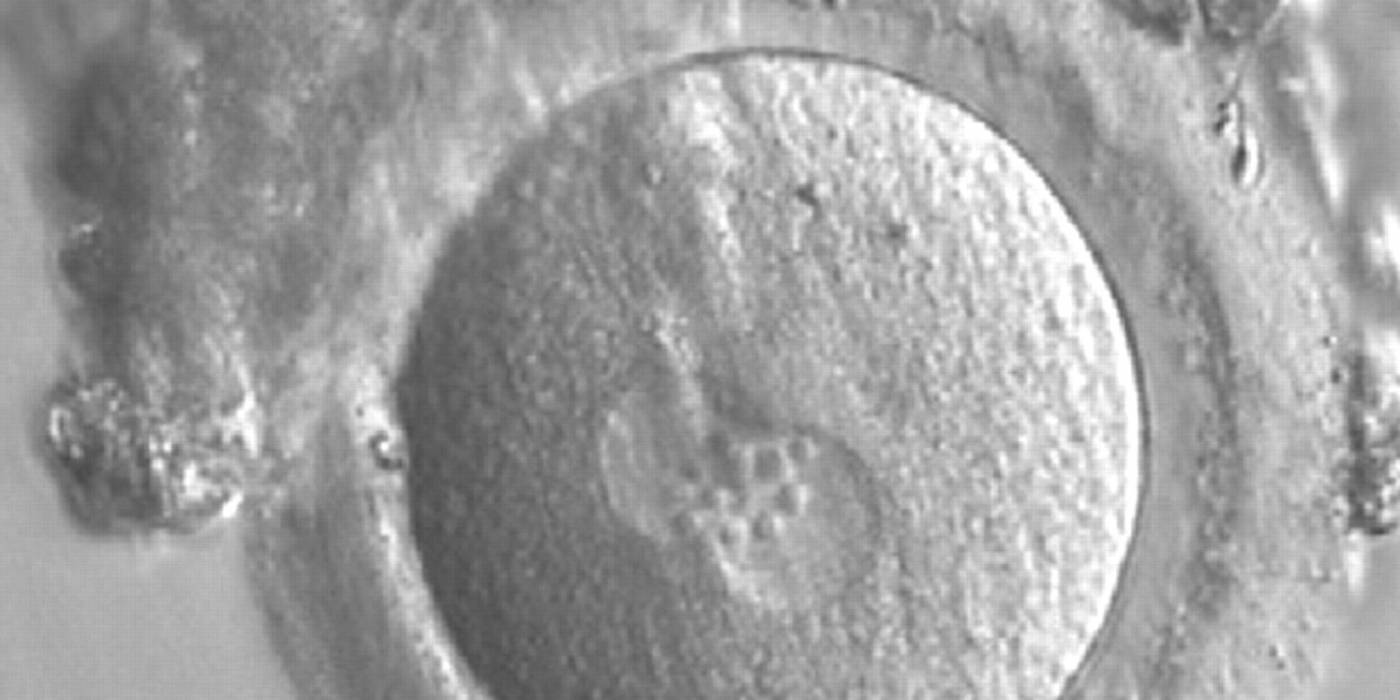
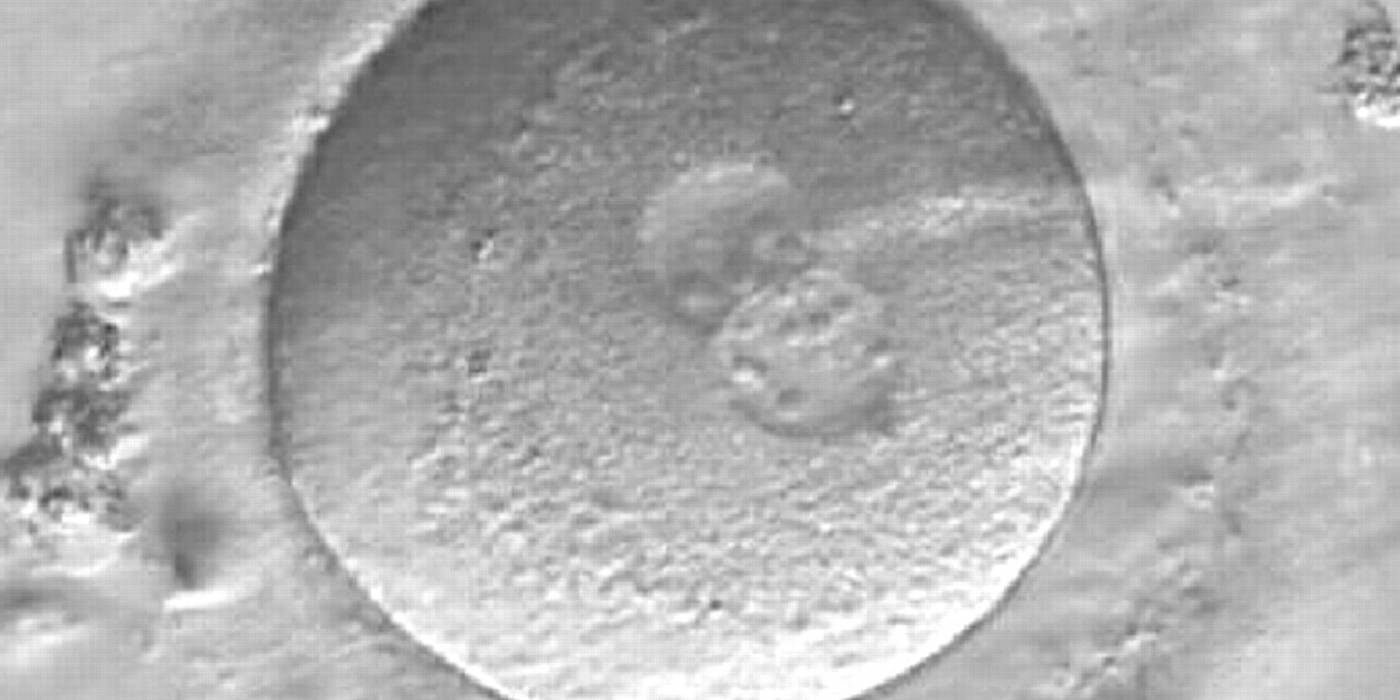
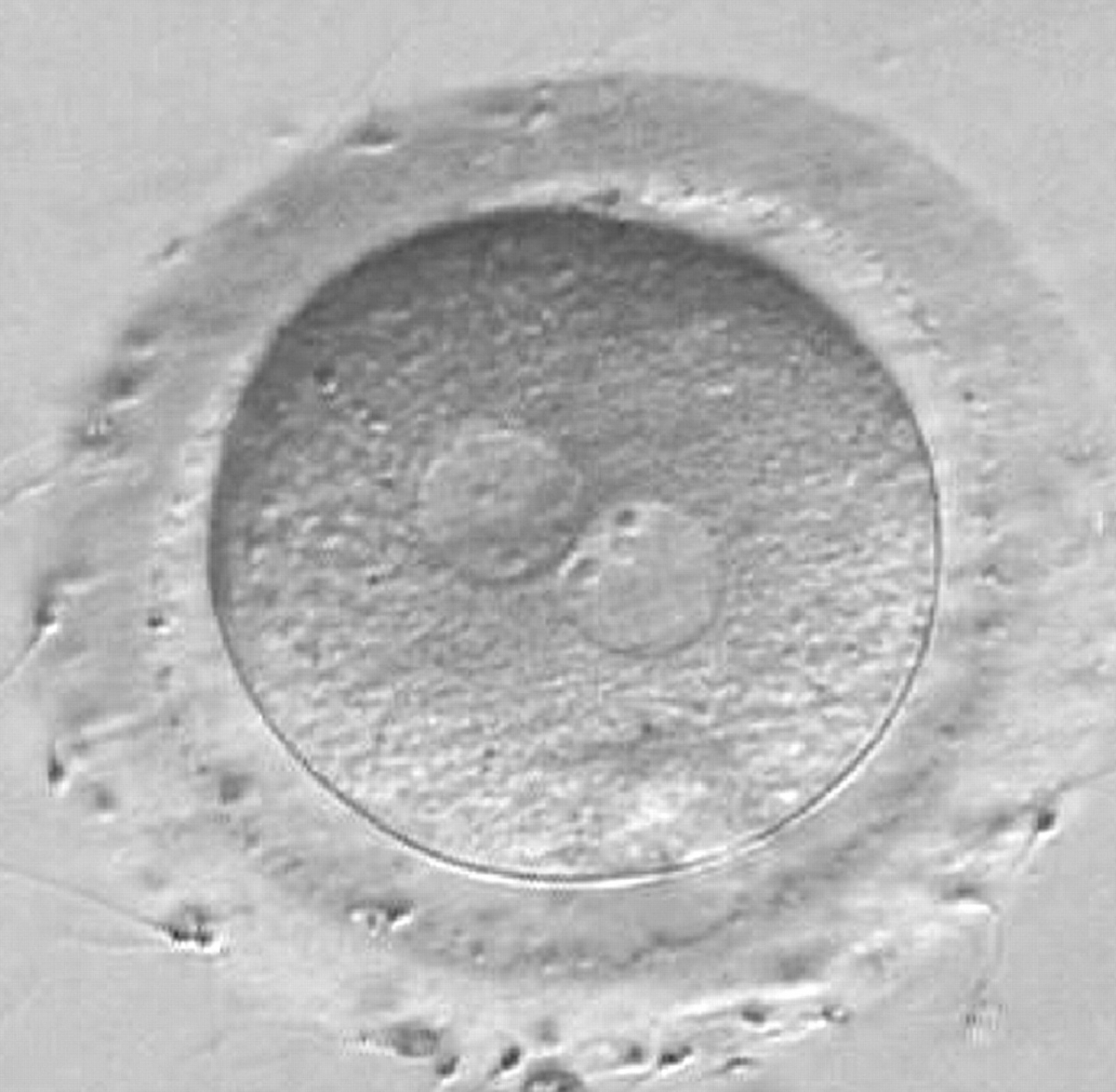
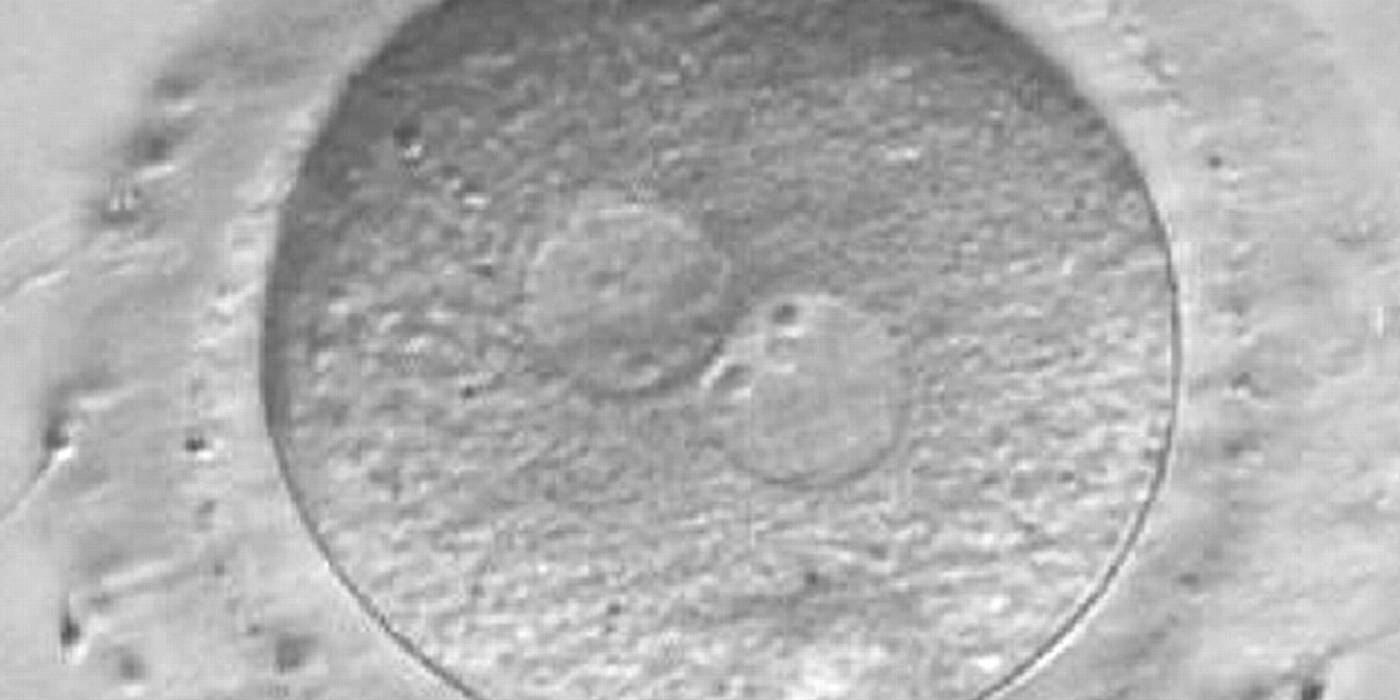
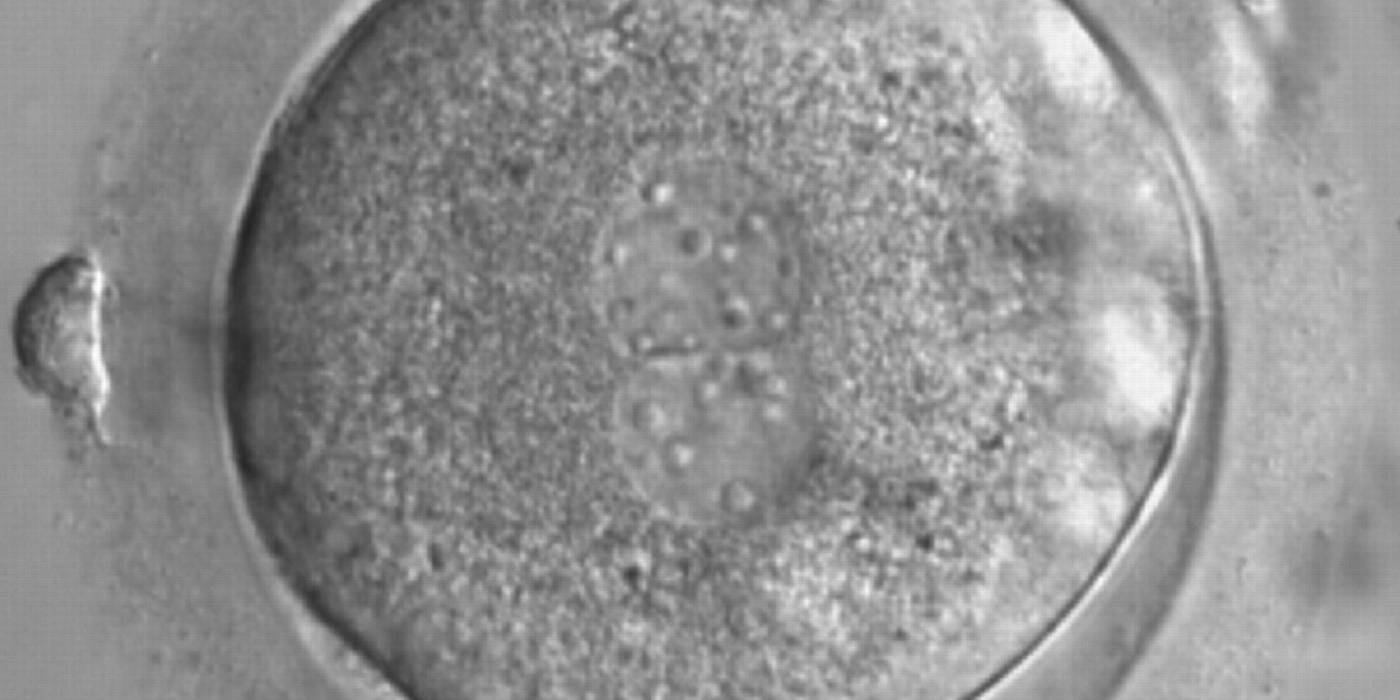
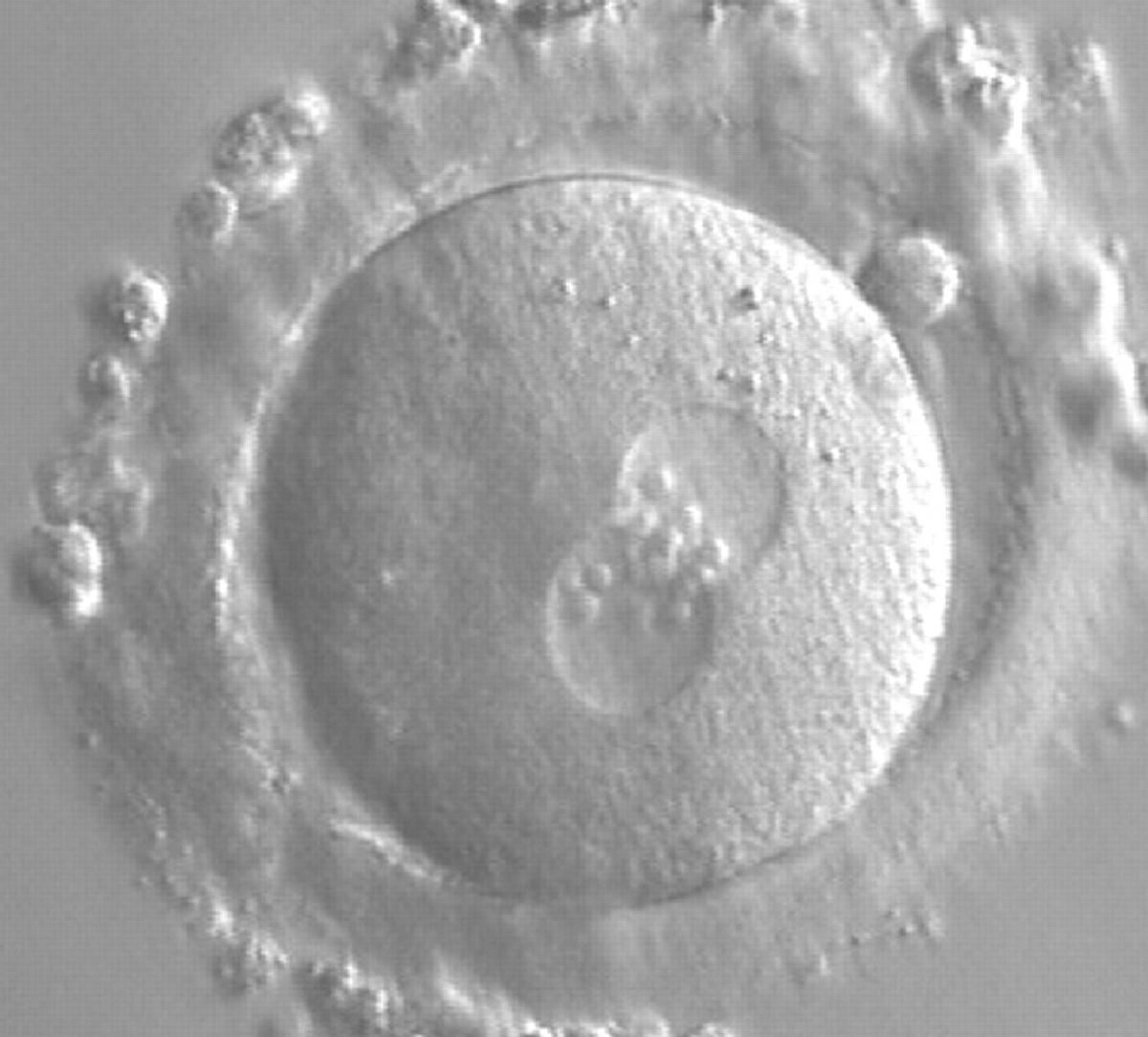
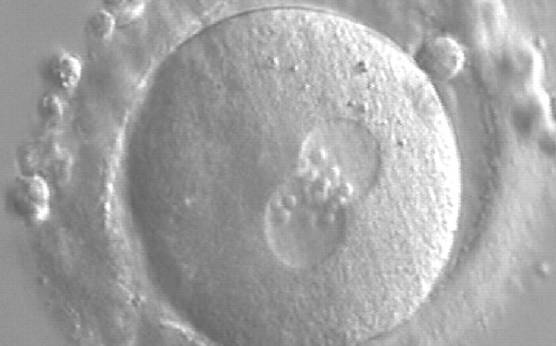
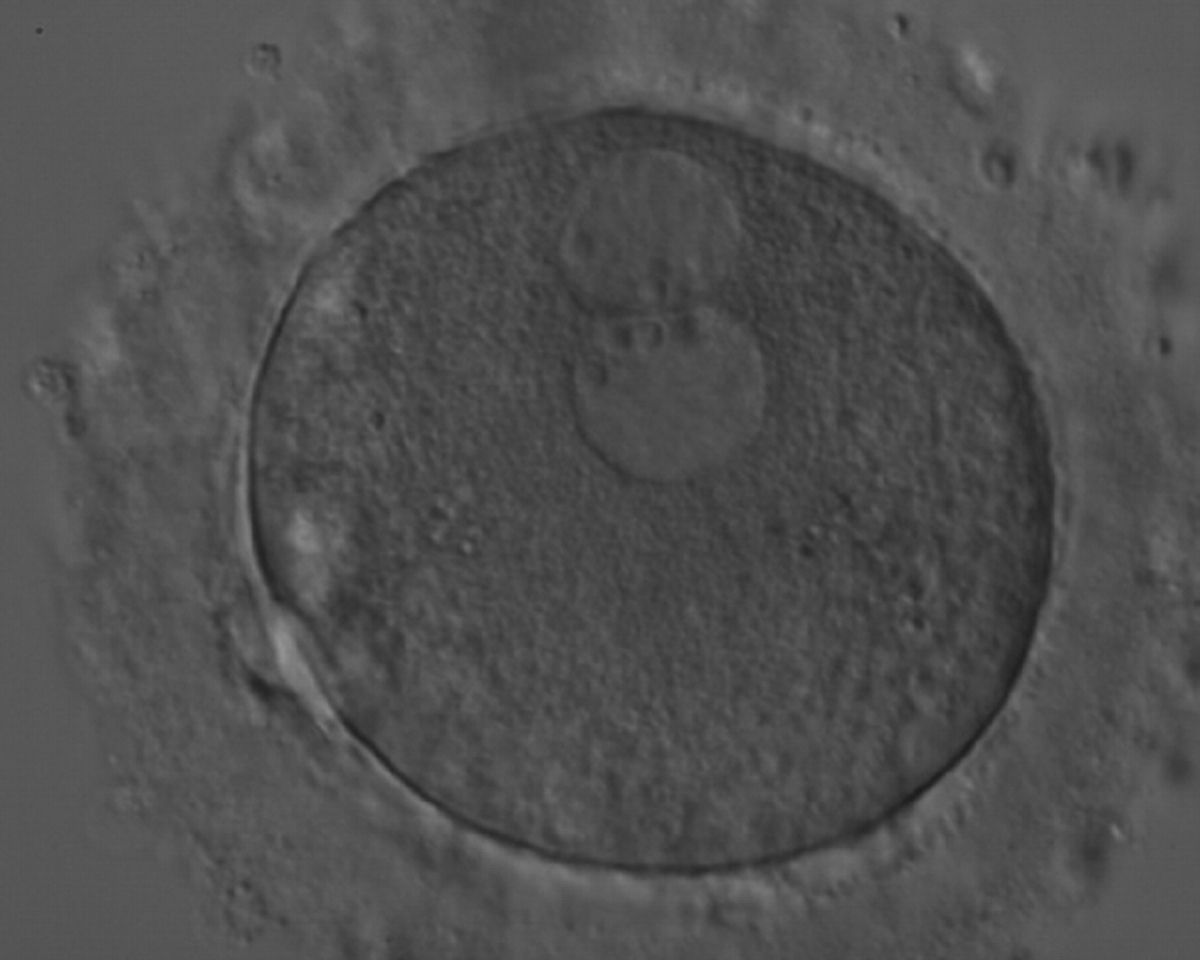
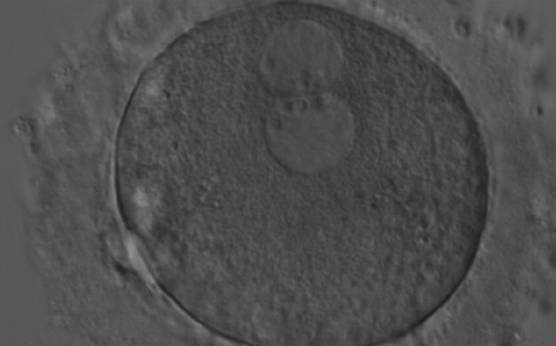
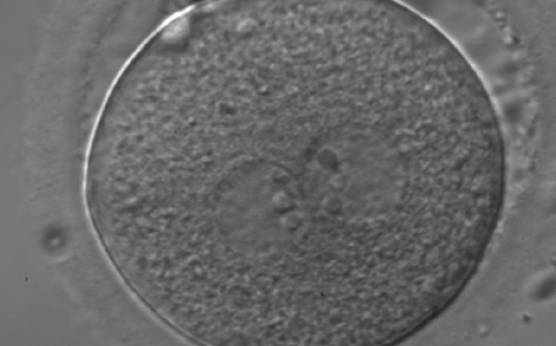
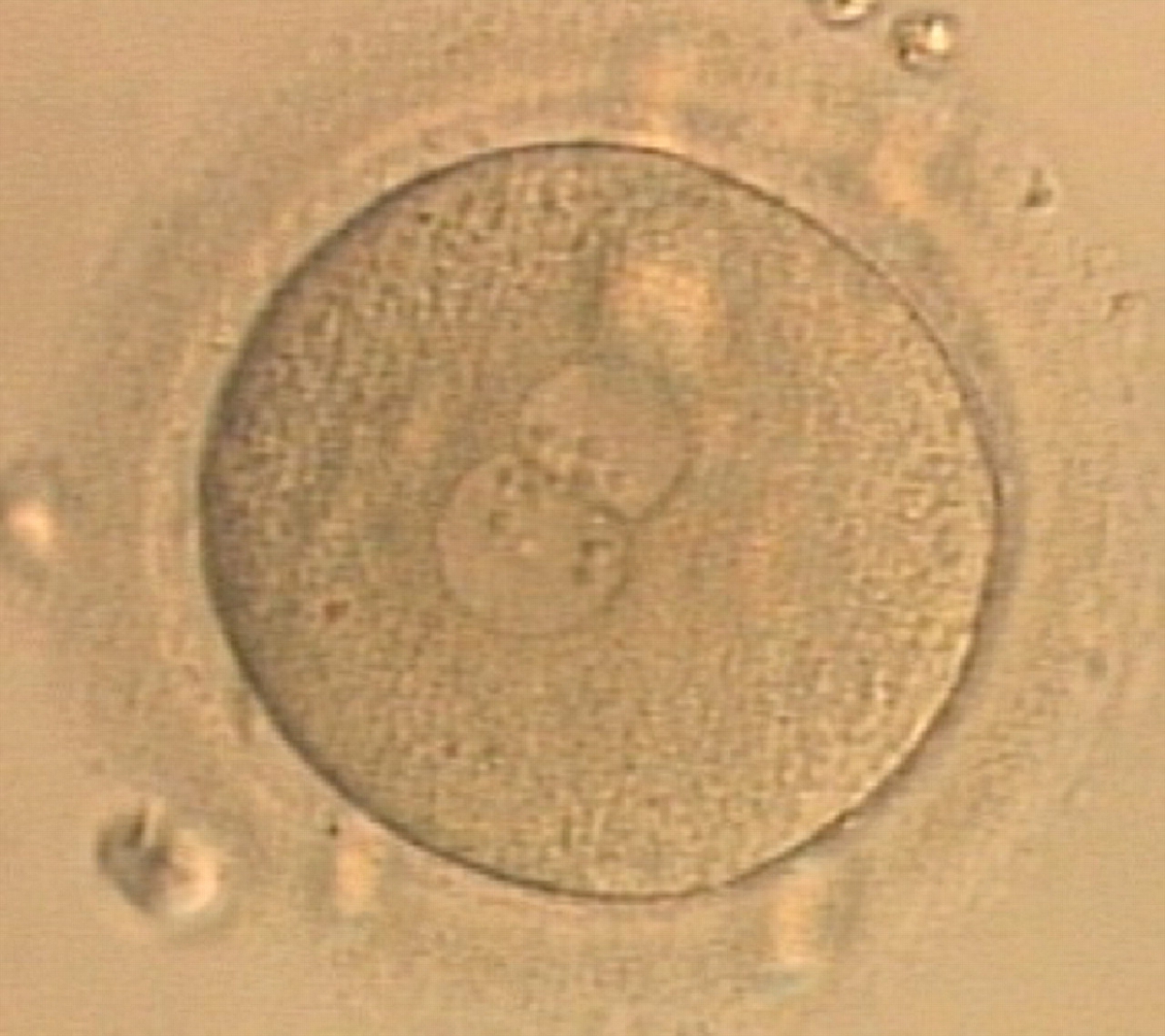
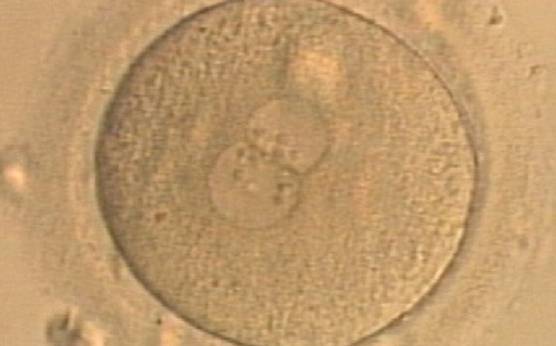
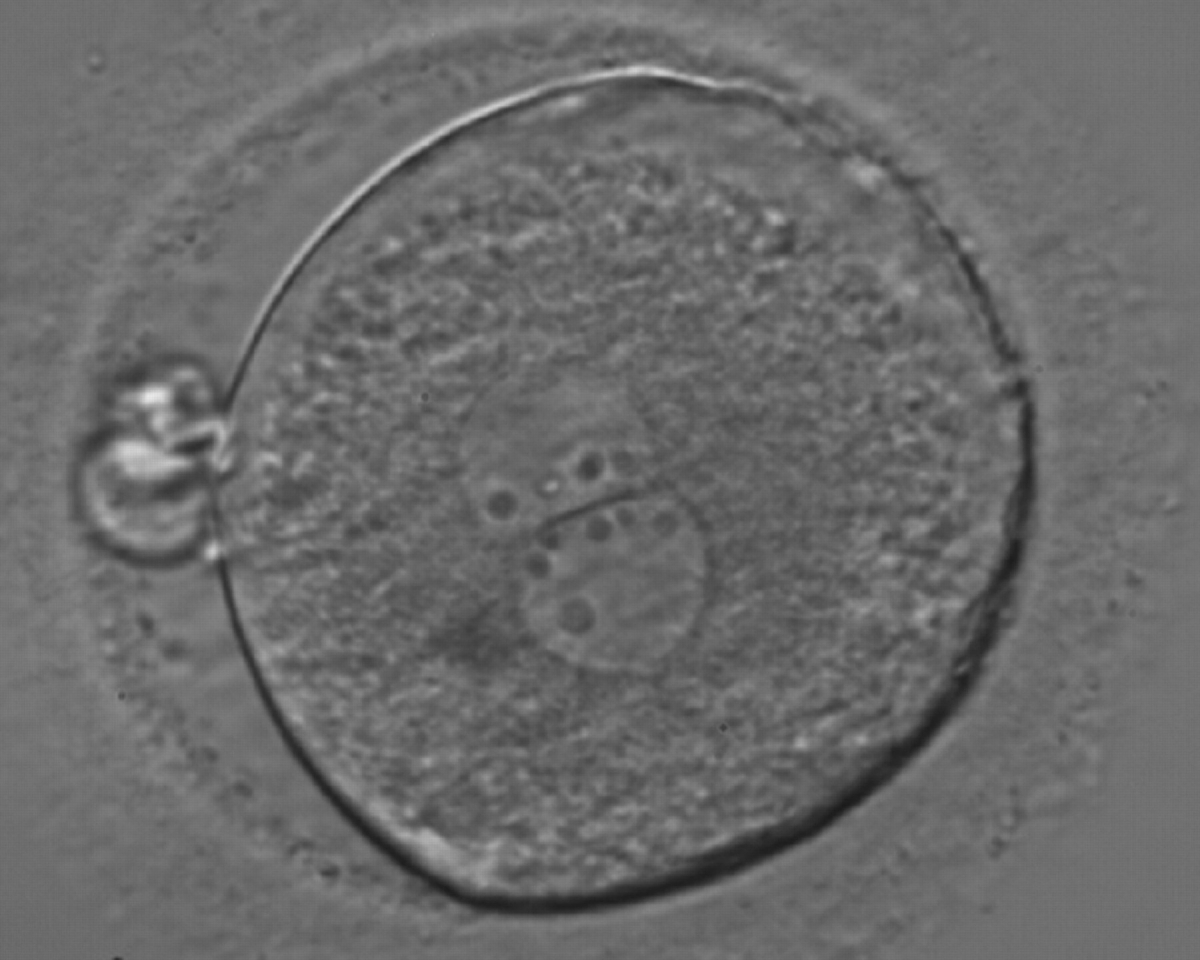
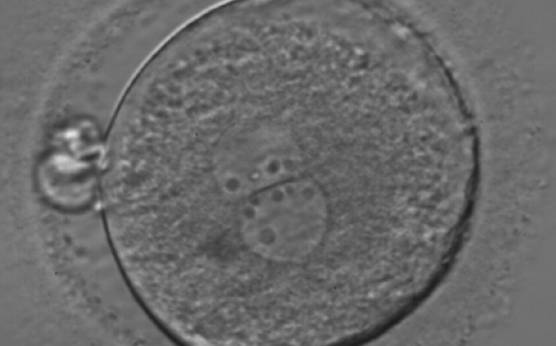
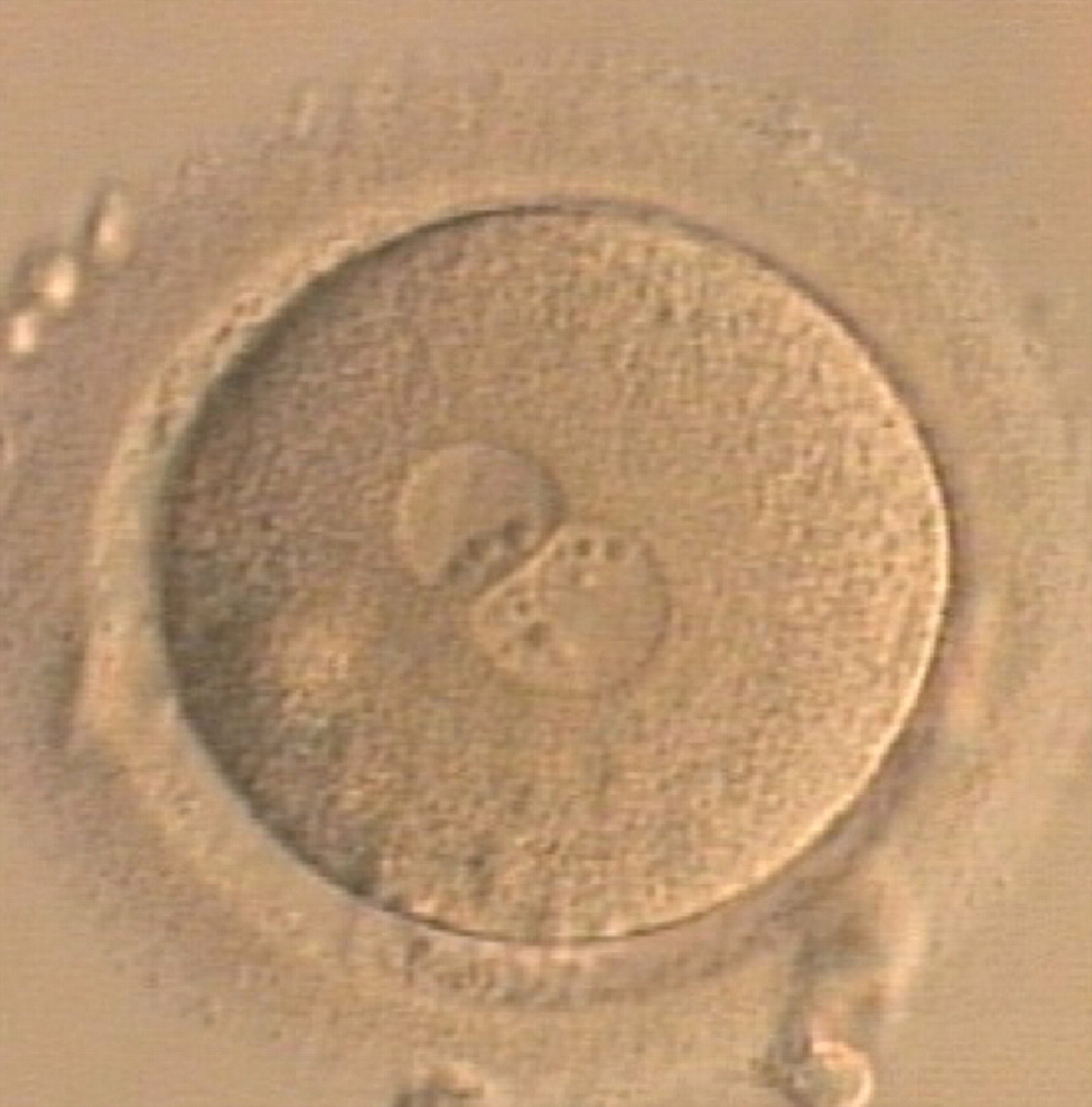
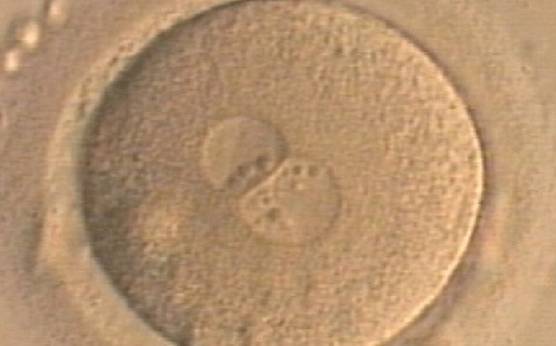
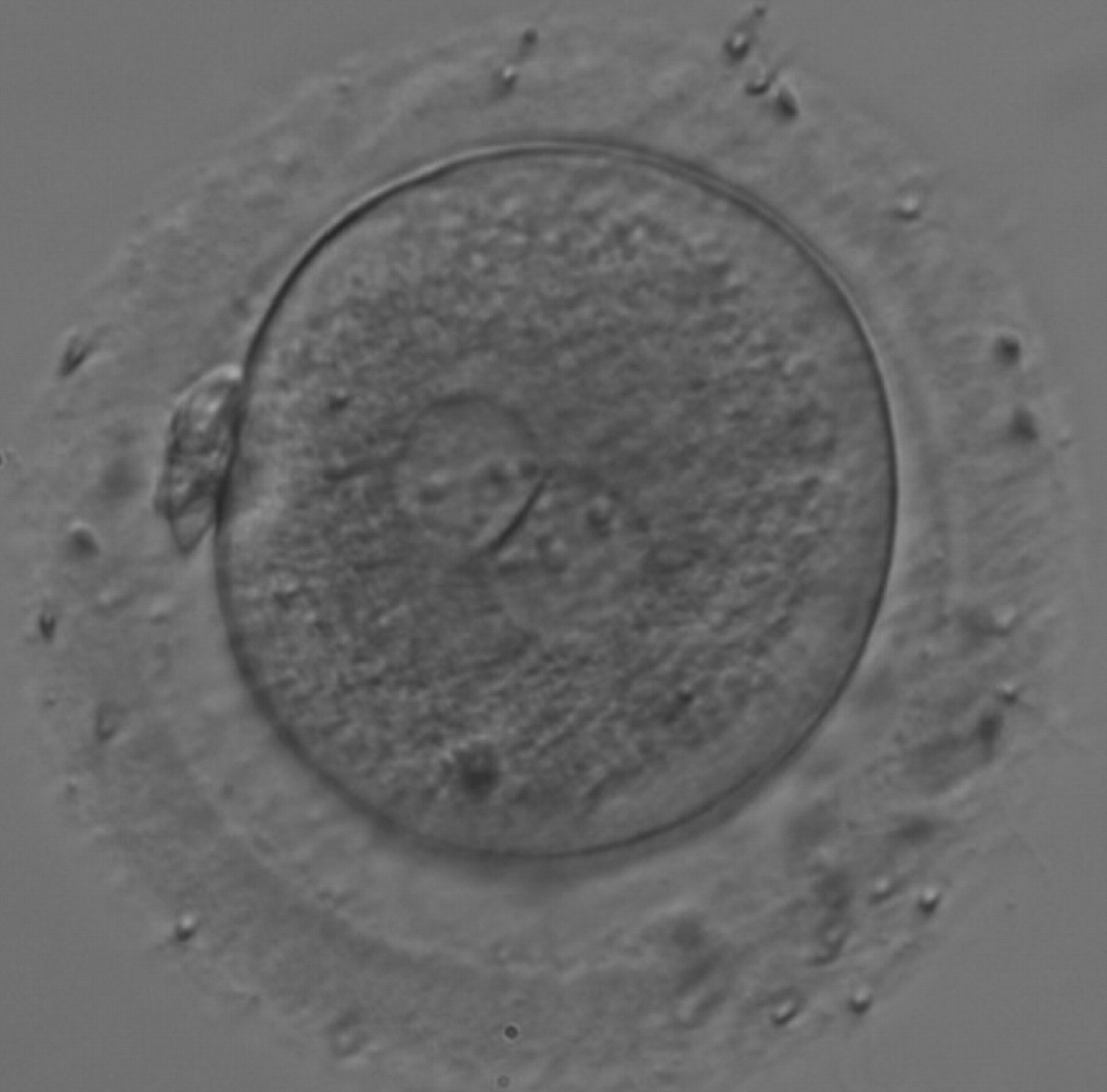
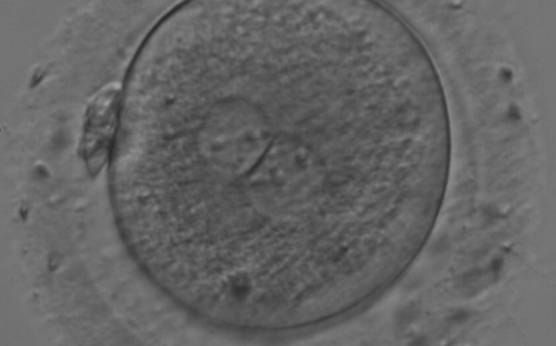
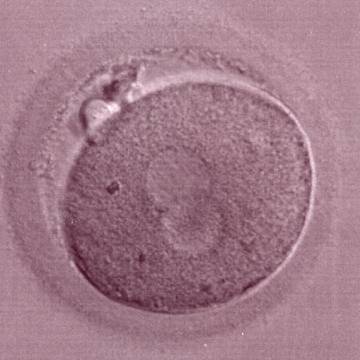
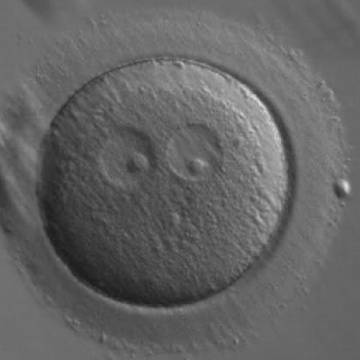
As PNs form after fertilization, there is polarized distribution in the chromatin into the furrow between them (Van Blerkom et al., 1997). As NPBs are attached to the chromatin, they should also polarize or align with it implying that, if there is correct chromatin polarization, the NPBs will appear polarized as well (Figs 151–154). Due to the dynamics of this event, some zygotes show symmetry in appearance of the PNs, but a delay in the alignment of the chromatin into the furrow, or onto the mitotic plate (Figs 155–157). NPB and PN progression can occur even in highly dysmorphic zygotes (Fig. 158).
During the progression of the cell cycle, NPBs change in number, size and distribution (Fig. 101) and finally they disappear shortly before syngamy and initiation of the first cleavage division (Fig. 149). Recent investigations by time-lapse imaging have shown that NPBs are highly dynamic and that a characteristic NPB pattern may change within a short period of time (Montag et al., 2011).
The potential use of the arrangement of NPBs in both PNs regarding size, number and symmetry was initially investigated as a major part of PN scoring in the late 90s (Scott and Smith, 1998). Several publications found a benefit of PN scoring and especially the distribution of NPBs with the outcome of assisted reproduction treatment (Tesarik and Greco, 1999; Scott et al., 2000; Balaban et al., 2001; Montag and van der Ven, 2001; Ebner et al., 2003; Senn et al., 2006). Others have reported no benefit (Payne et al., 2005; James et al., 2006; Nicoli et al., 2007; Brezinova et al., 2009).
NPBs' morphology is influenced by the patient's age and there is also a temporal difference in morphology between zygotes from IVF compared with ICSI. Therefore, comparative PN/NPB scoring should be performed according to strict guidelines considering timing, patient characteristics and insemination technique employed.
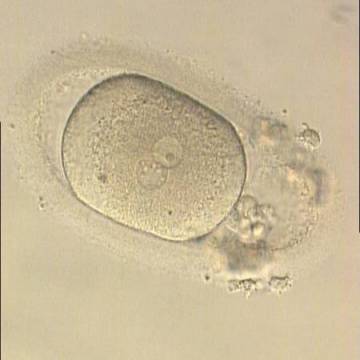
Many cell cycle control proteins are located in the nucleolus, and it has been shown in mitotic cells that asymmetry in number and pattern of NPBs is associated with abnormal cell cycles and ultimately with abnormal development (Pedersen, 1998). It is plausible that asymmetry between PNs in zygotes (Figs 159–162) can lead to abnormal development with an increase in fragmentation and abnormal cleavage, and reduced viability (Scott, 2003). Nevertheless, implantation can occur (Figs 163 and 164).
Pronuclear scoring based on Scott's (2003) Z scores, which combines the assessment of PN orientation and NPB pattern, was adopted by the consensus workshop (Alpha Scientists in Reproductive medicine and ESHRE Special Interest Group of Embryology, 2011) but modified into three categories:
- symmetrical PNs (corresponding to Scott's Z1 and Z2)
- non-symmetrical PNs (other arrangements, peripheral PNs)
- abnormal PNs (PNs with none or one NPB, which are called respectively ‘ghost’ PNs and ‘bulls-eye’ PNs. Both have been associated with abnormal outcome in animal models.
D.2 Numbers similar/numbers different between PNs
Human cells generally have two to seven nucleoli per human nucleus with equal numbers in the two daughter cells after a mitotic division. Nucleoli appear and disappear depending on the cell cycle phase: they are more numerous at the G1 phase, then start to fuse, and at the S1 phase there are only one to two large nucleoli per nucleus. When asynchrony occurs, this appears to be the result of aberrant chromosomal function.
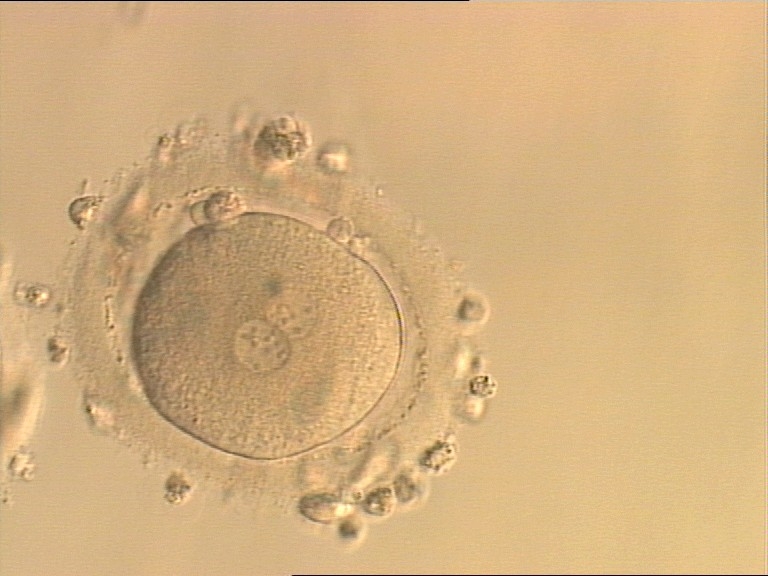
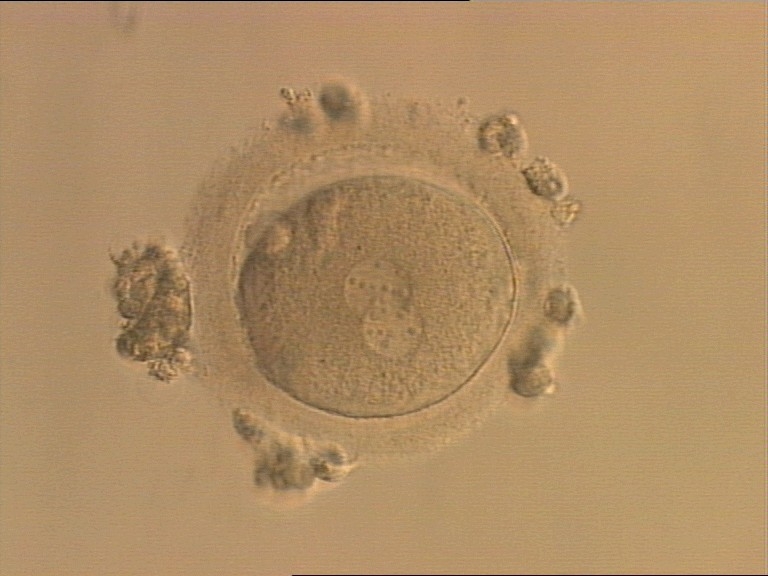
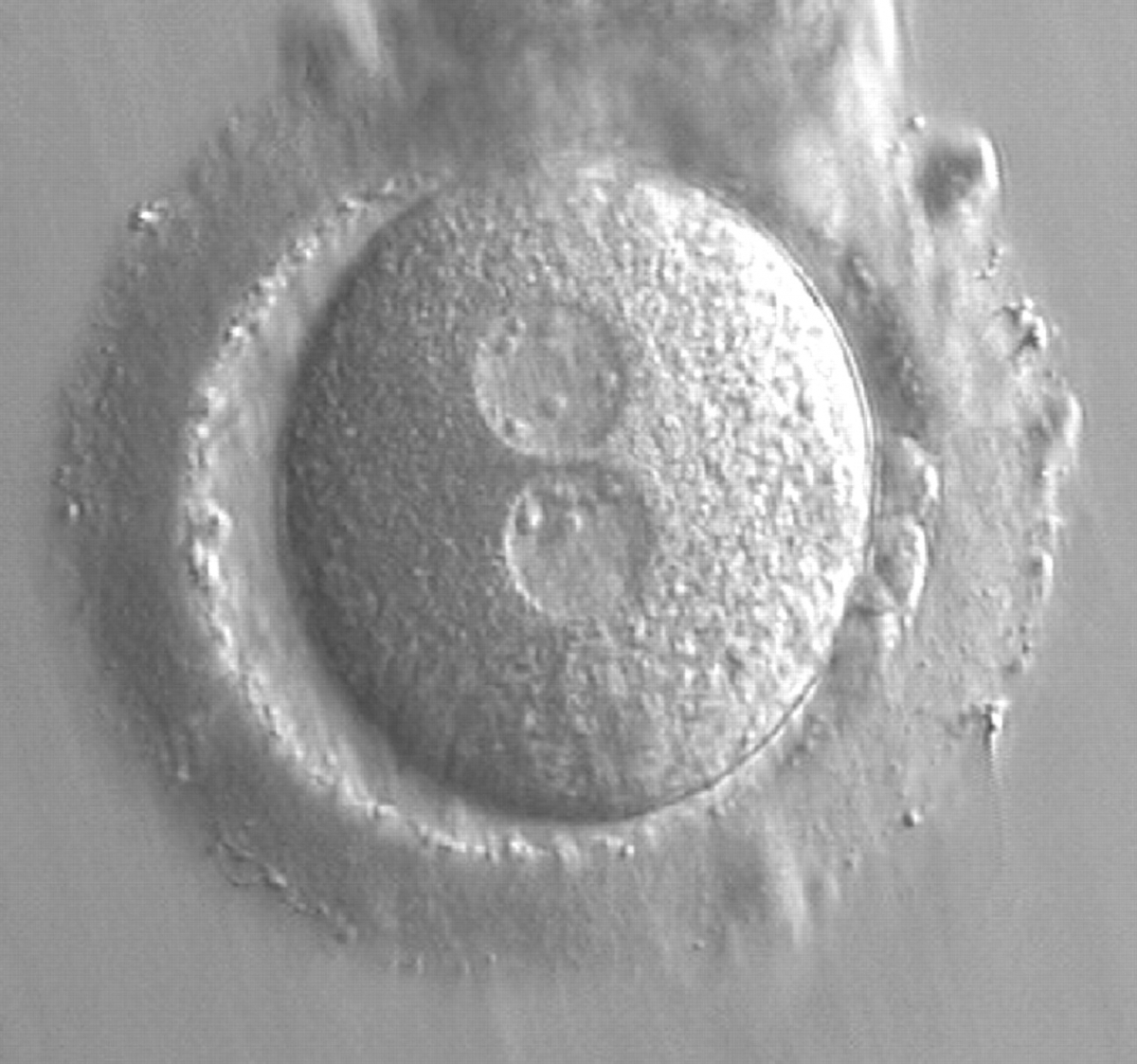
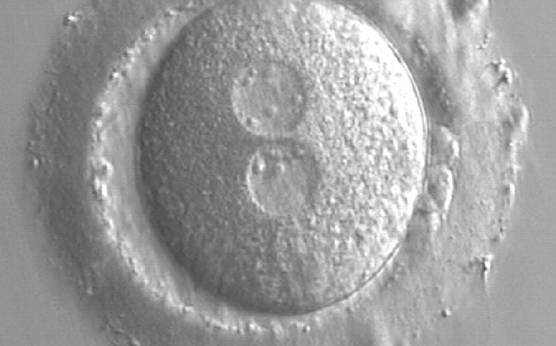
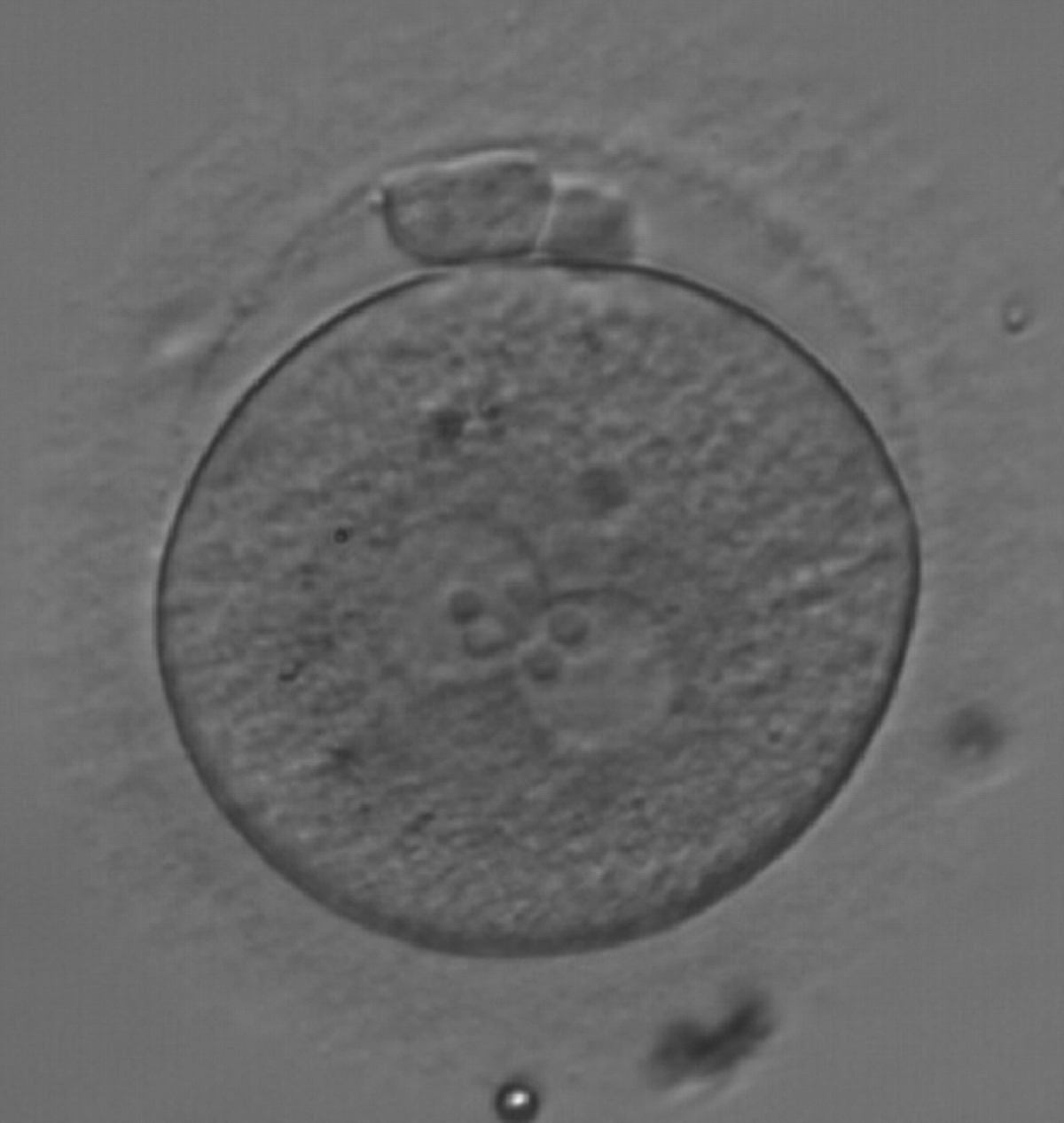
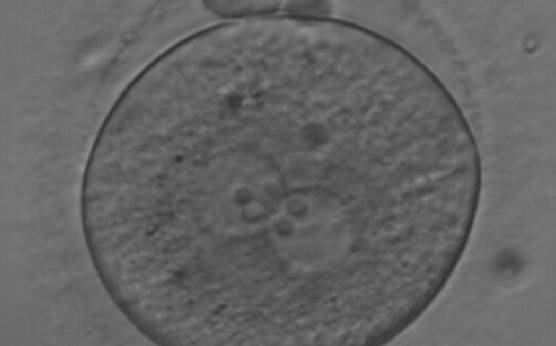
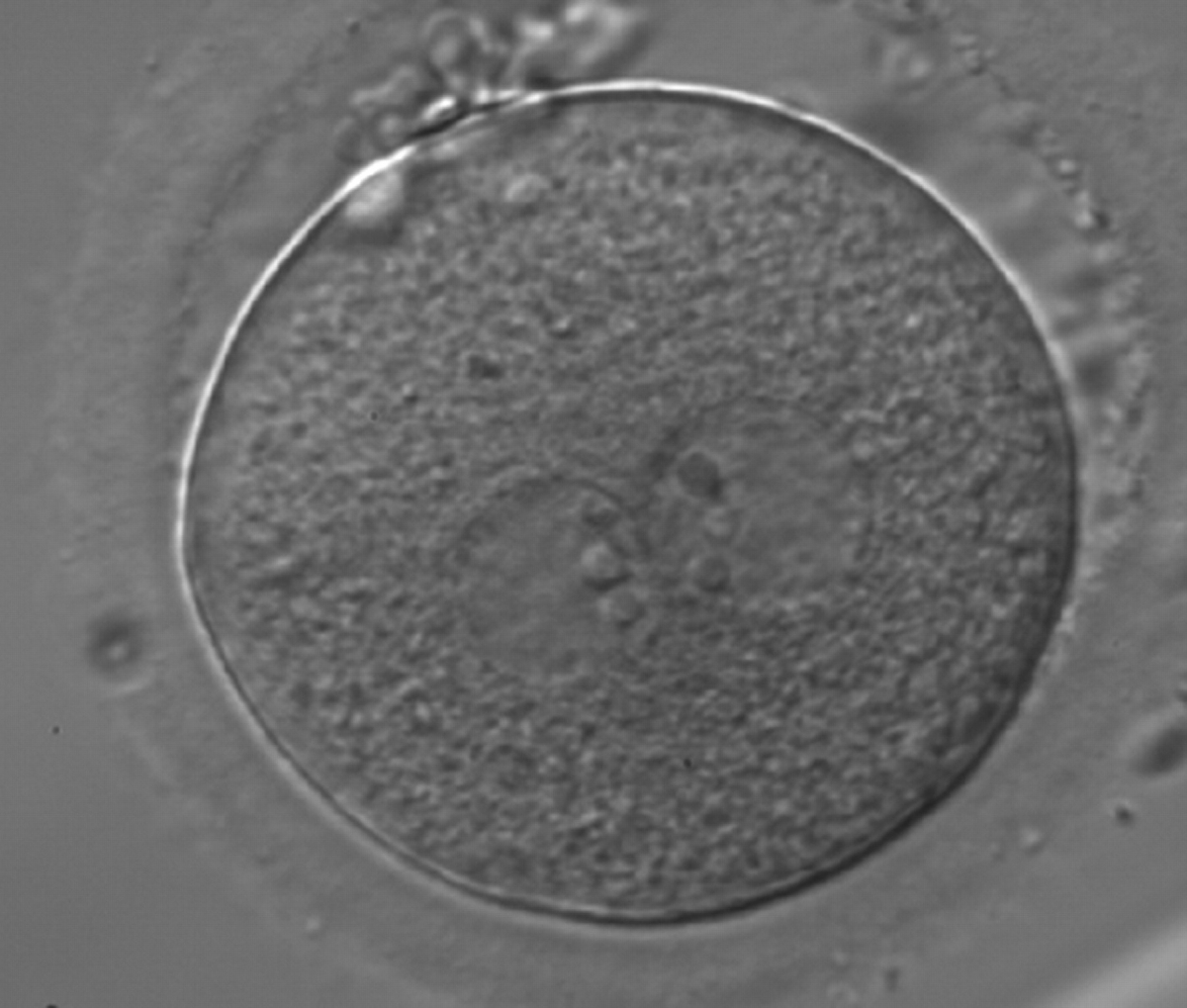
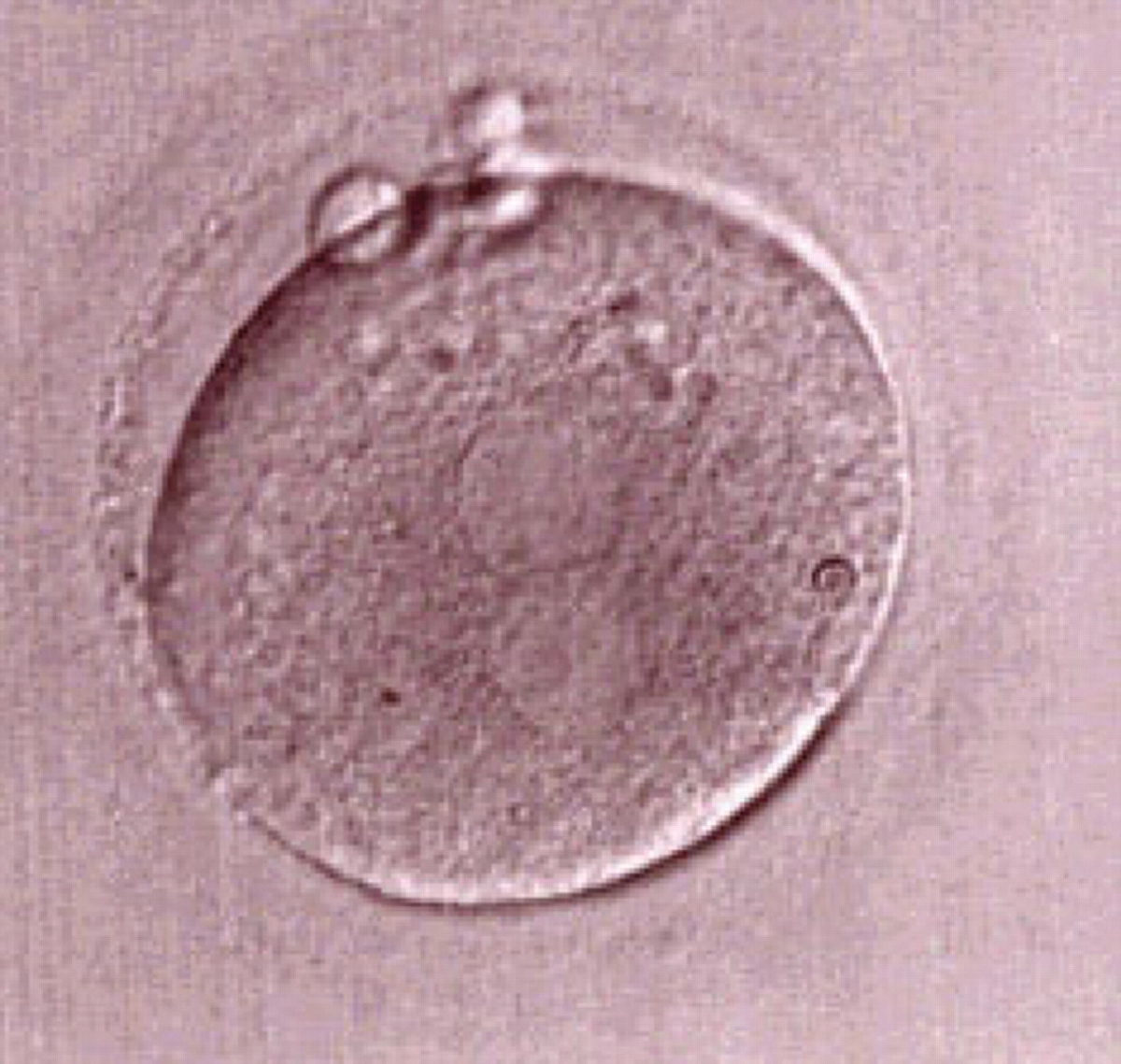
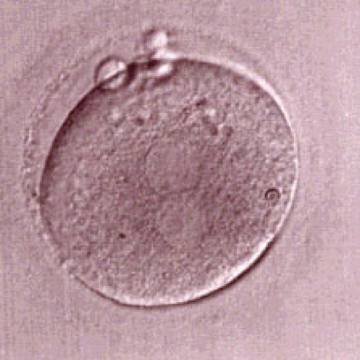
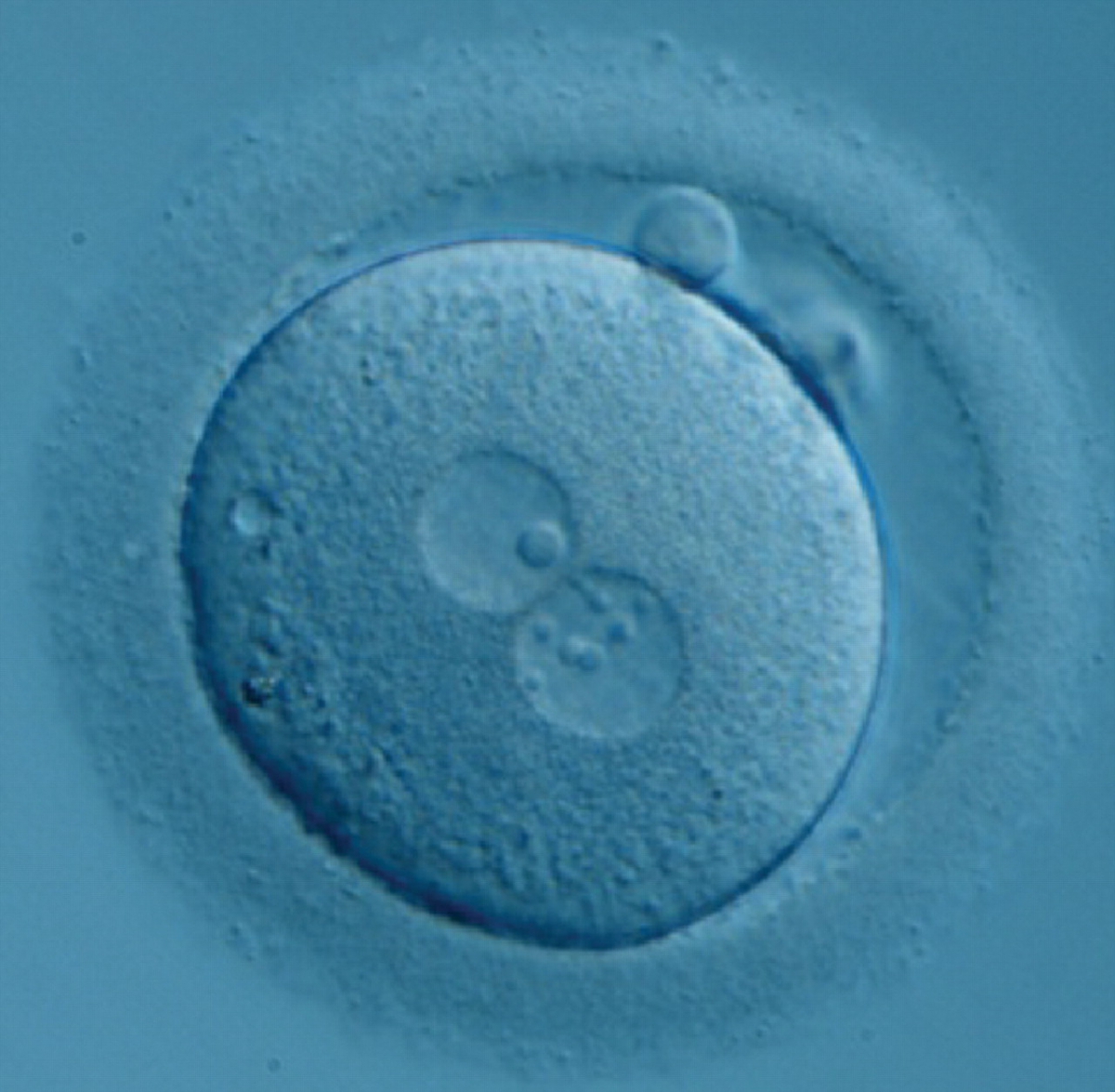
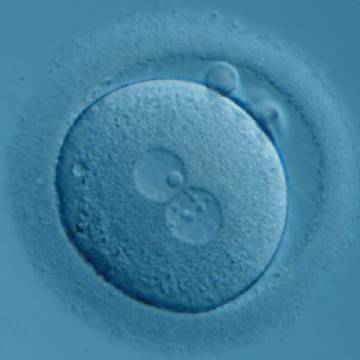
Transferring this model to the zygote, the ideal oocytes are those presenting with symmetry for number and size of NPBs that are aligned on the furrow between the 2PNs (Figs 165–167). Equality in number with non-alignment in both PNs is also indicative of synchronised development (Fig. 168). In contrast, any form of disparity in NPBs' size, number or pattern of alignment between the PNs, is associated with a poor outcome (Figs 169–172).
D.3 Similar size of NPBs/different size of NPBs
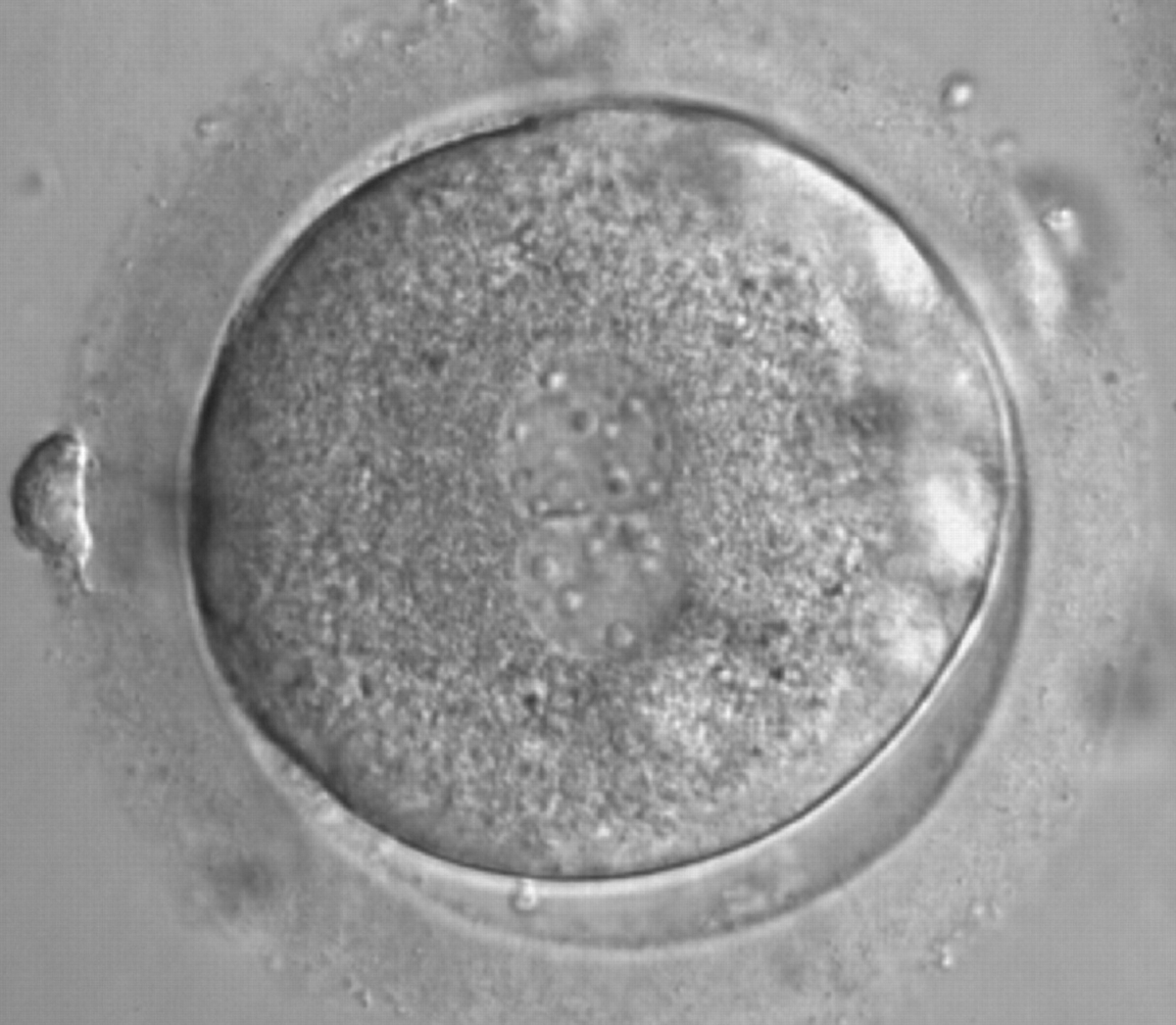
The dynamics of nucleoli formation associated with NPBs merging implies a time-related change in NPBs' size (Scott, 2003). A progressive increase in NPBs' size and a concomitant reduction in number occur at the time of nucleoli alignment into the furrow between PNs (Figs 173–176).
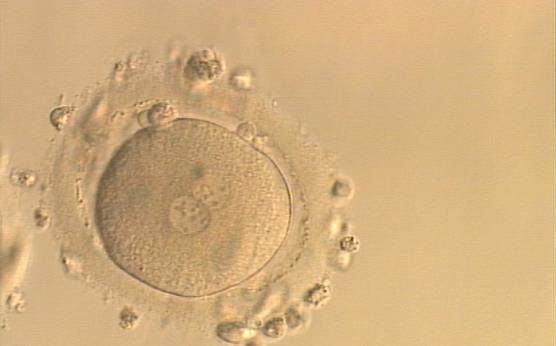
The presence of small scattered and unequal-sized NPBs (Figs 177–179) could be indicative of functional defects in nucleoli with consequent decreased or ineffective synthesis of rRNA. The extent of malfunctioning possibly depends on the grade of asynchrony and on the number of affected nucleoli as demonstrated by the clinical outcome that may result in implantation (Fig. 180).
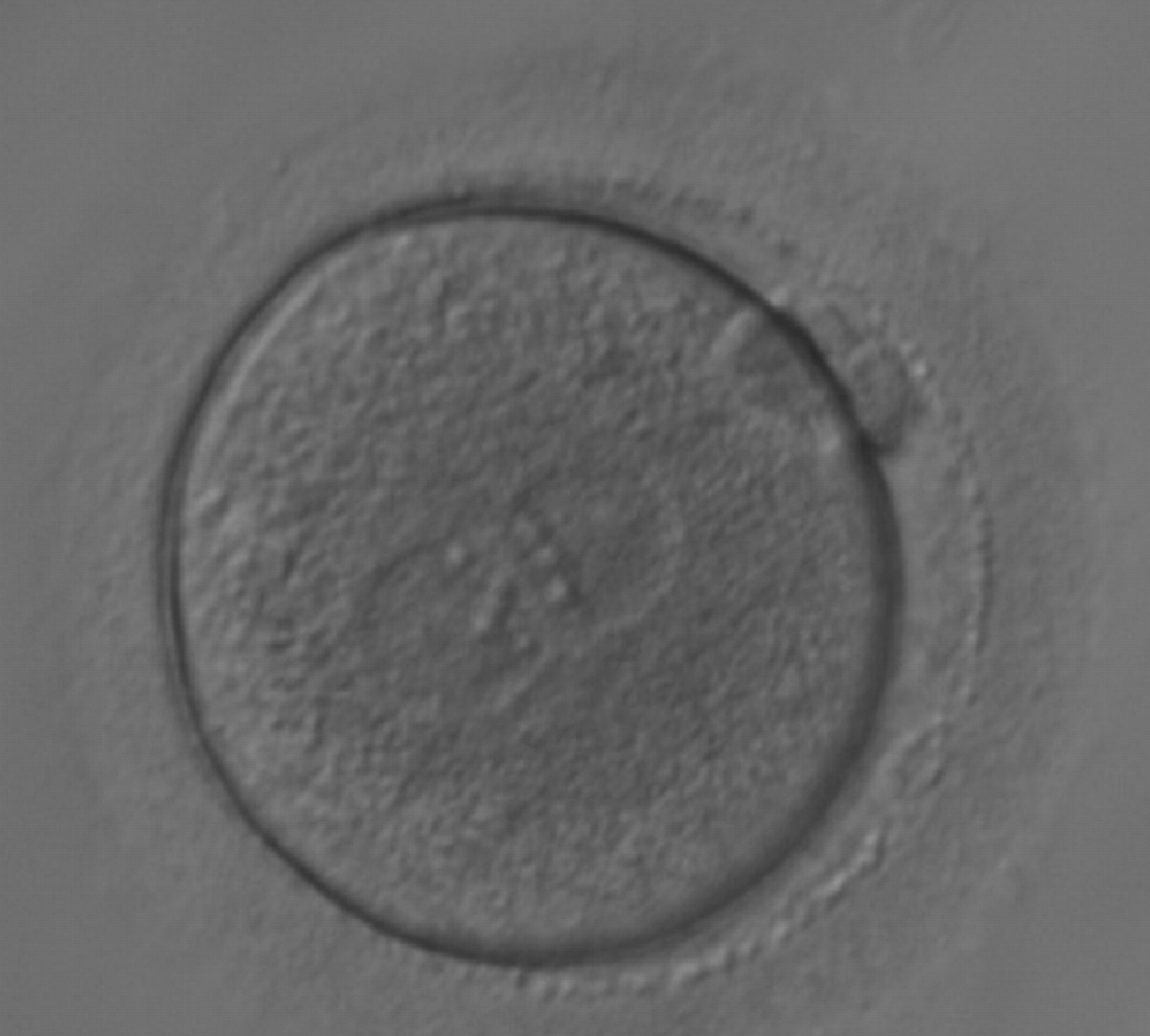
D.4 Ghost PNs (absent NPBs)/single NPB in one or more PNs (bull’s eye)
Despite the difficulty of making comparative studies aimed at evaluating the relevance of PN scoring, the sequence of processes involved in fertilization underlines the importance and significance of NPBs and nucleoli in determining embryo viability. In light of these considerations, it is not surprising that the absence of nucleoli in PNs at the time of fertilization check, the so-called ‘ghost’ PNs (Figs 181–184), or the presence of a single NPB, known as ‘bull's eye’ PNs (Figs 185–189) has been reported to be associated with epigenetic defects and abnormal development in animals (Svarcova et al., 2009).
Article references:
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 2011;26:1270-1283.
Abstract/FREE Full Text
Balaban B, Urman B, Isiklar A, Alatas C, Aksoy S, Mercan R, Mumcu A, Nuhoglu A. The effect of pronuclear morphology on embryo quality parameters and blastocyst transfer outcome. Hum Reprod 2001;16:2357-2361.
Abstract/FREE Full Text
Brezinova J, Oborna I, Svobodova M, Fingerova H. Evaluation of day one embryo quality and IVF outcome—a comparison of two scoring systems. Reprod Biol Endocrinol 2009;7:9.
CrossRef | Medline | Google Scholar
Ebner T, Moser M, Sommergruber M, Gaiswinkler U, Wiesinger R, Puchner M, Tews G. Presence, but not type or degree of extension, of a cytoplasmic halo has a significant influence on preimplantation development and implantation behaviour. Hum Reprod 2003;18:2406-2412.
Abstract/FREE Full Text
Edwards RG, Beard HK. Oocyte polarity and cell determination in early mammalian embryos. Mol Hum Reprod 1997;3:863-905.
Abstract/FREE Full Text
James AN, Hennessy S, Reggio B, Wiemer K, Larsen F, Cohen J. The limited importance of pronuclear scoring of human zygotes. Hum Reprod 2006;21:1599-1604.
Abstract/FREE Full Text
Montag M, van der Ven H. Evaluation of pronuclear morphology as the only selection criterion for further embryo culture and transfer: results of a prospective multicentre study. Hum Reprod 2001;16:2384-2389.
Abstract/FREE Full Text
Montag M, Liebenthron J, Köster M. Which morphological scoring system is relevant in human embryo development? Placenta 2011;32:S252-S256.
CrossRef | Medline | Web of Science | Google Scholar
Nicoli A, Valli B, Di Girolamo R, Di Tommaso B, Gallinelli A, La Sala GB. Limited importance of pre-embryo pronuclear morphology (zygote score) in assisted reproduction outcome in the absence of embryo cryopreservation. Fertil Steril 2007;88:1167-1173.
CrossRef | Medline | Web of Science | Google Scholar
Payne D, Flaherty SP, Barry MF, Matthews CD. Preliminary observations on polar body extrusion and pronuclear formation in human oocytes using time-lapse video cinematography. Hum Reprod 1997;12:532-541.
Abstract/FREE Full Text
Payne JF, Raburn DJ, Couchman GM, Price TM, Jamison MG, Walmer DK. Relationship between pre-embryo pronuclear morphology (zygote score) and standard day 2 or 3 embryo morphology with regard to assisted reproductive technique outcomes. Fertil Steril 2005;84:900-909.
CrossRef | Medline | Web of Science | Google Scholar
Pedersen T. Growth factors in the nucleolus? J Cell Biol 1998;143:279-281.
FREE Full Text
Scott LA, Smith S. The successful use of pronuclear embryo transfers the day following oocyte retrieval. Hum Reprod 1998;13:1003-1013.
Abstract/FREE Full Text
Scott L, Alvero R, Leondires M, Miller B. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod 2000;15:2394-2403.
Abstract/FREE Full Text
Scott L. Oocyte and embryo polarity. Semin Reprod Med 2001;18:171-183.
Google Scholar
Scott L. Pronuclear scoring as a predictor of embryo development. Reprod Biomed Online 2003;6:201-214.
CrossRef | Medline | Google Scholar
Senn A, Urner F, Chanson A, Primi MP, Wirthner DW, Germond M. Morphological scoring of human pronuclear zygotes for prediction of pregnancy outcome. Hum Reprod 2006;21:234-239.
Abstract/FREE Full Text
Svarcova O, Dinnyes A, Polgar Z, Bodo S, Adorjan M, Meng Q, Maddox-Hyttel P. Nucleolar re-activation is delayed in mouse embryos cloned from two different cell lines. Mol Reprod Dev 2009;76:132-141.
CrossRef | Medline | Web of Science | Google Scholar
Tesarik J, Greco E. The probability of abnormal preimplantation development can be predicted by a single static observation on pronuclear stage morphology. Hum Reprod 1999;14:1318-1323.
Abstract/FREE Full Text
Van Blerkom J, Antczak M, Schrader R. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod 1997;12:1047-1055.
Abstract/FREE Full Text