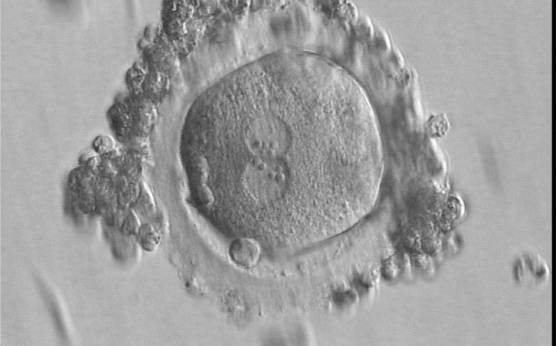
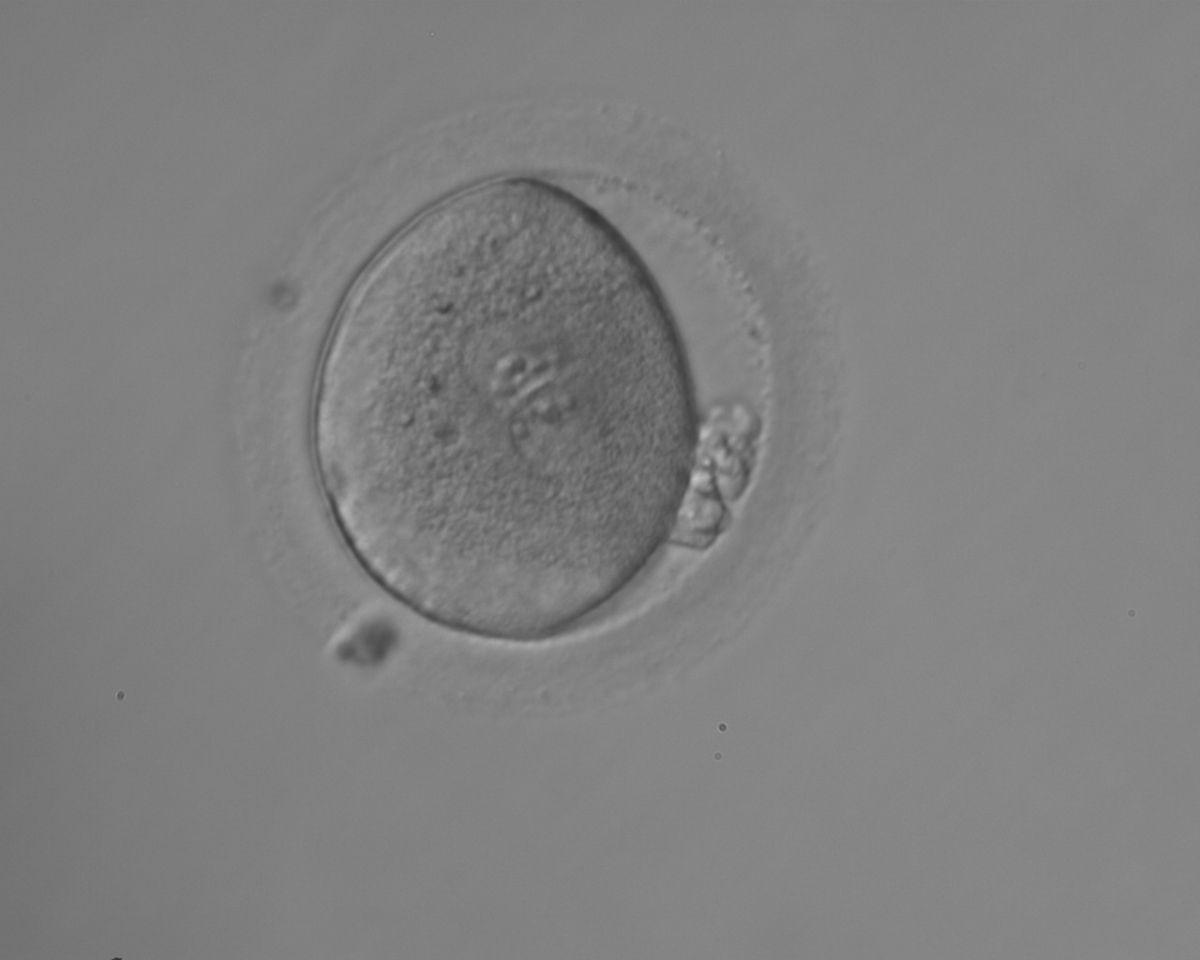
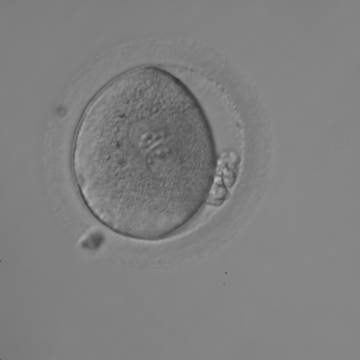
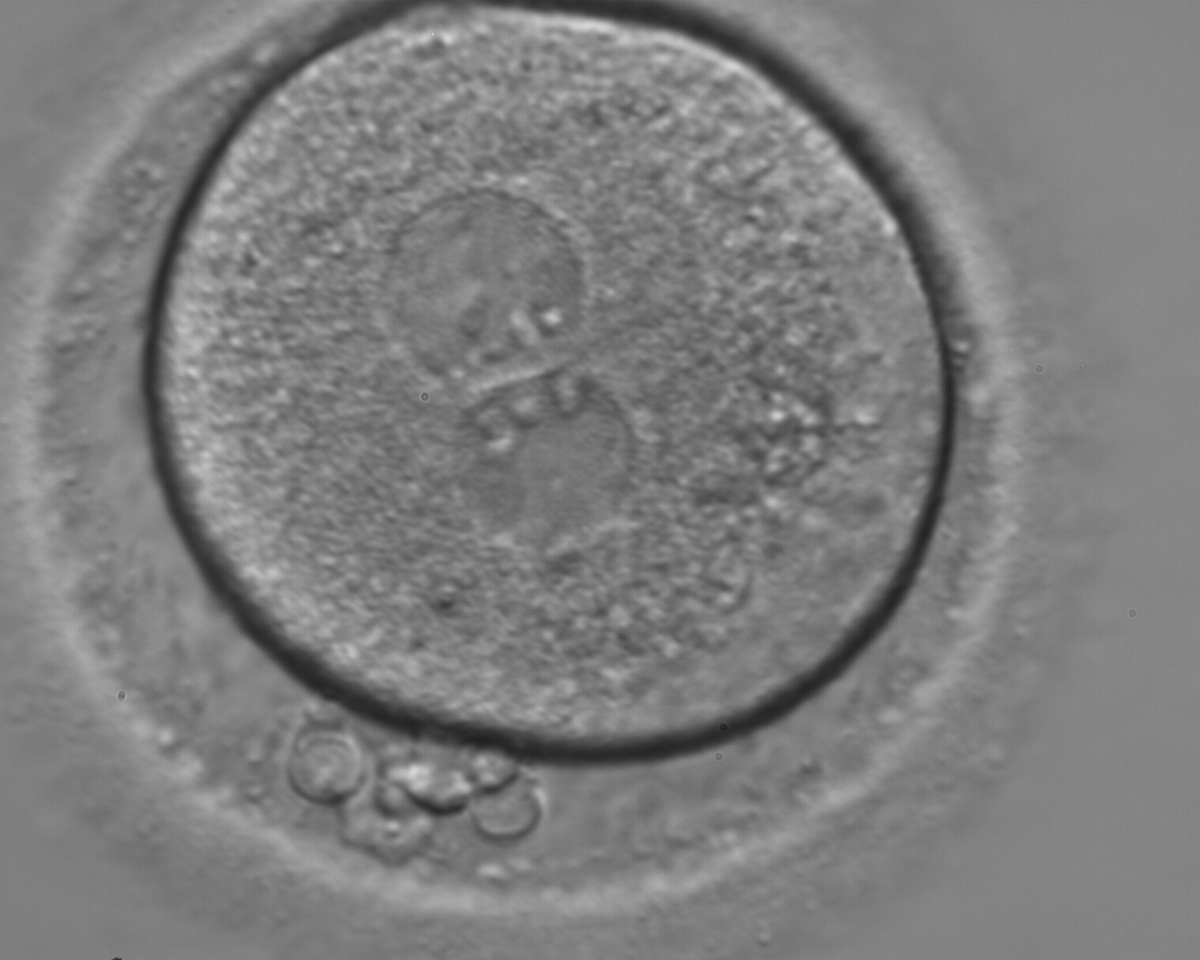
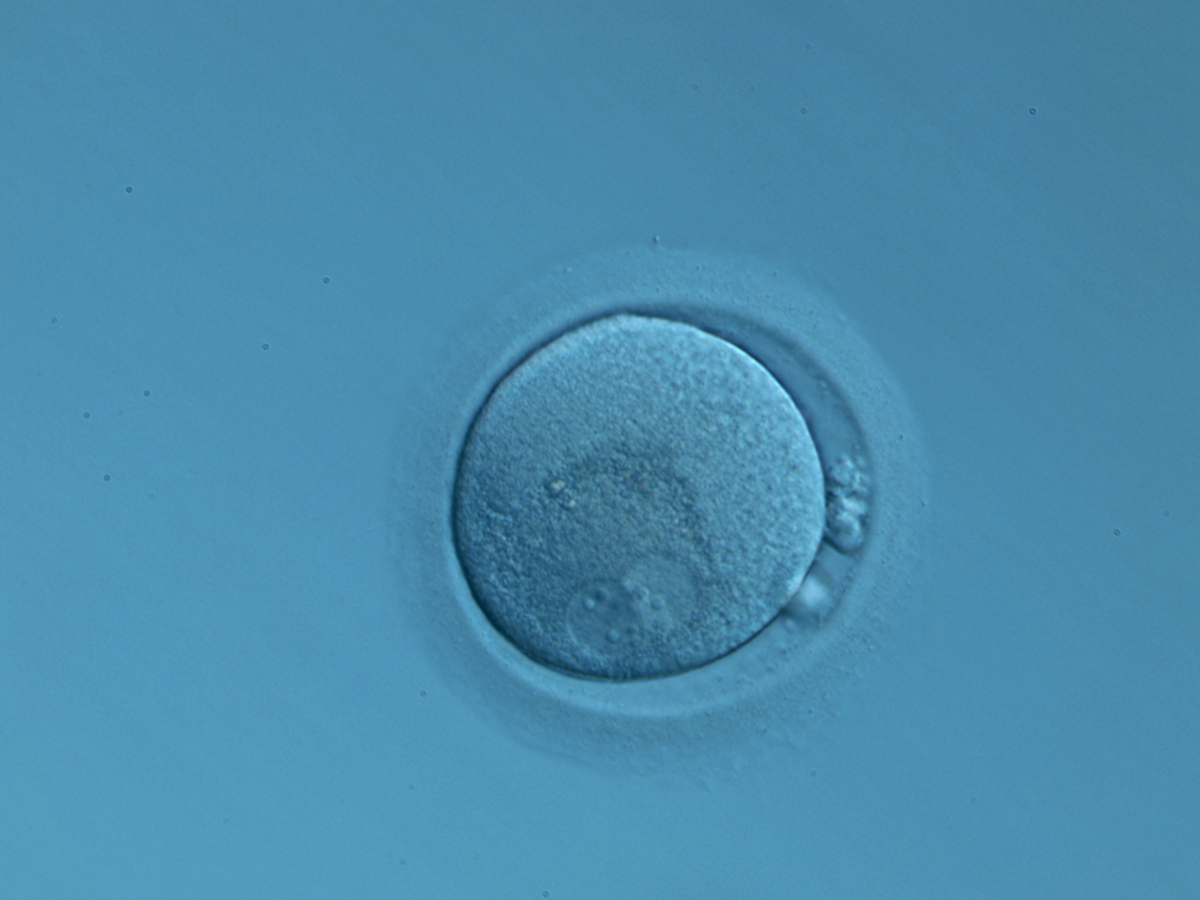
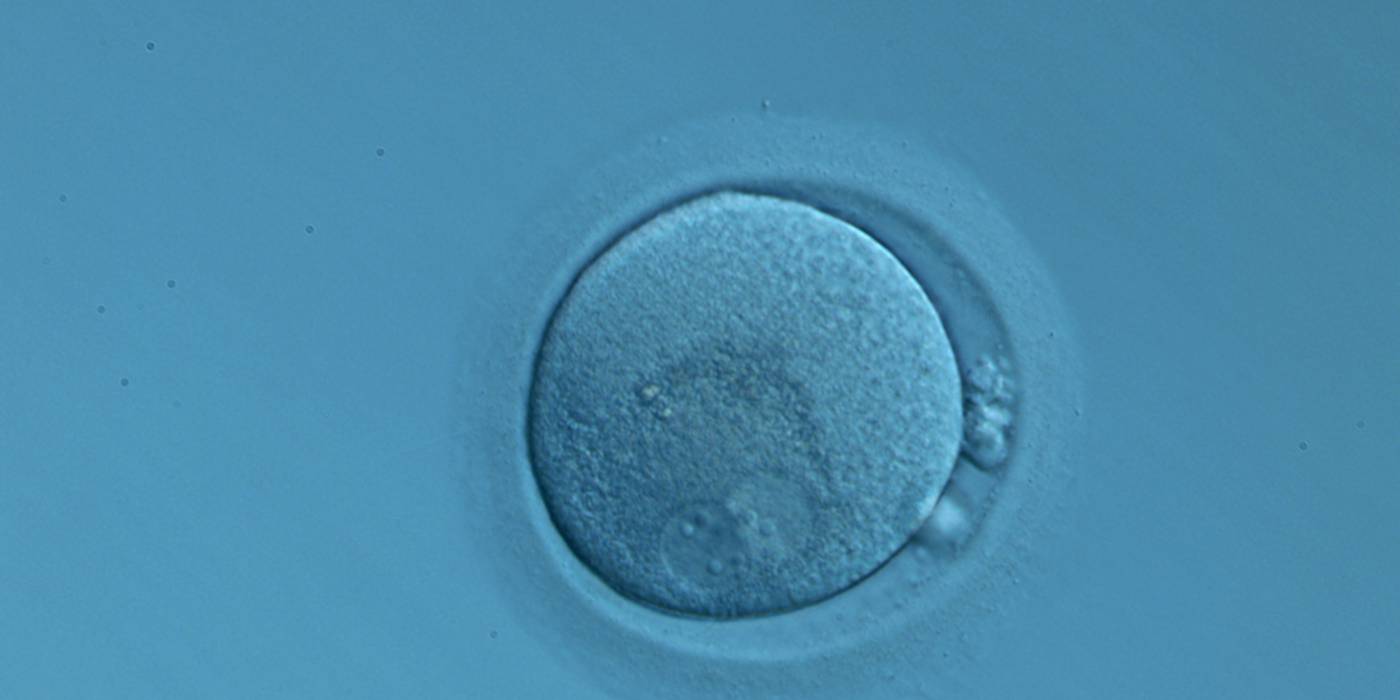
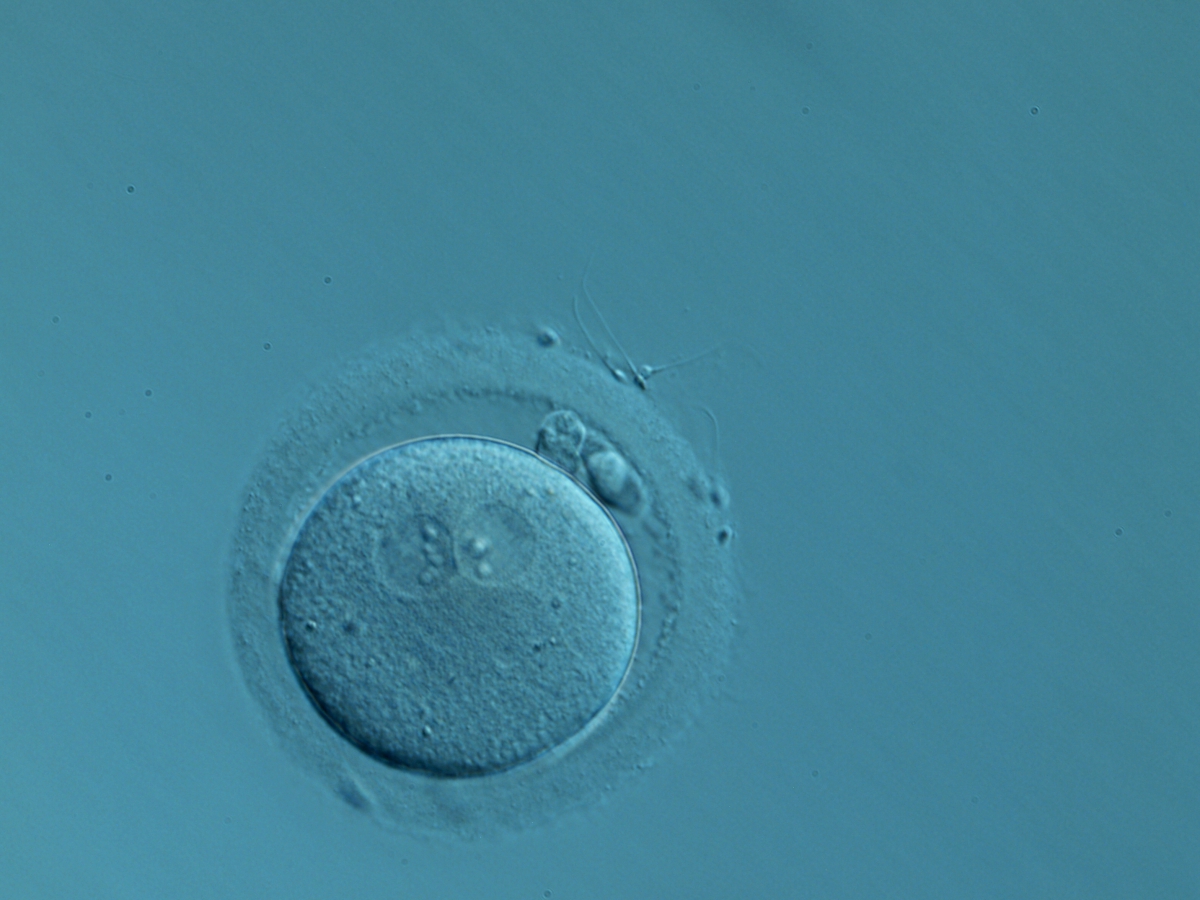
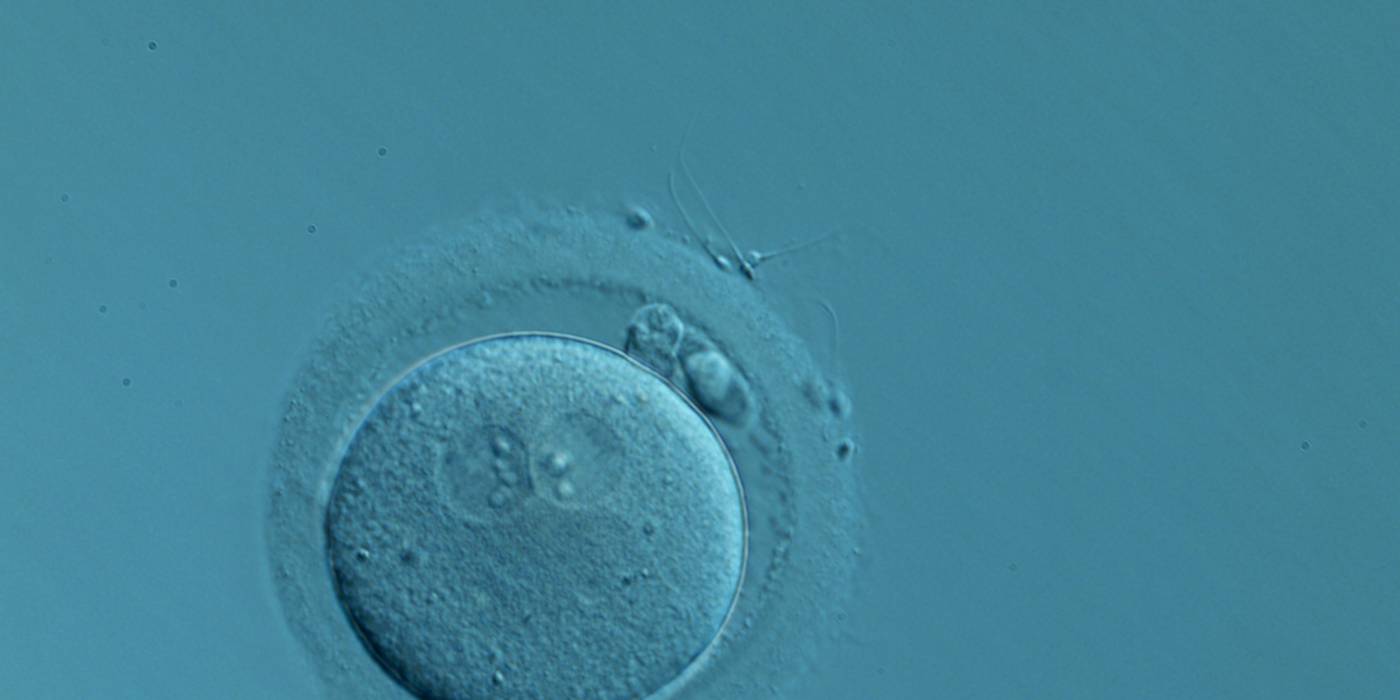
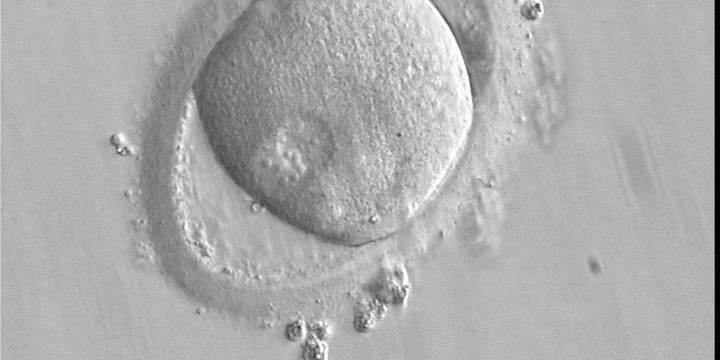
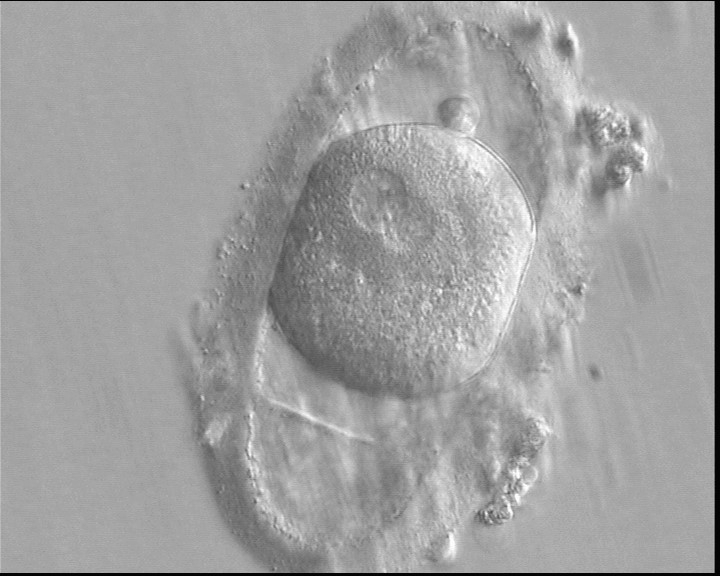
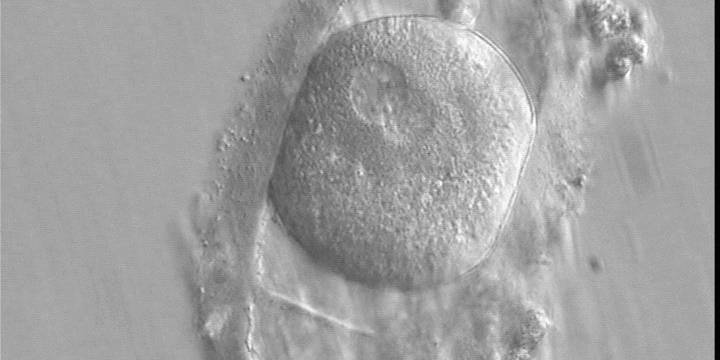
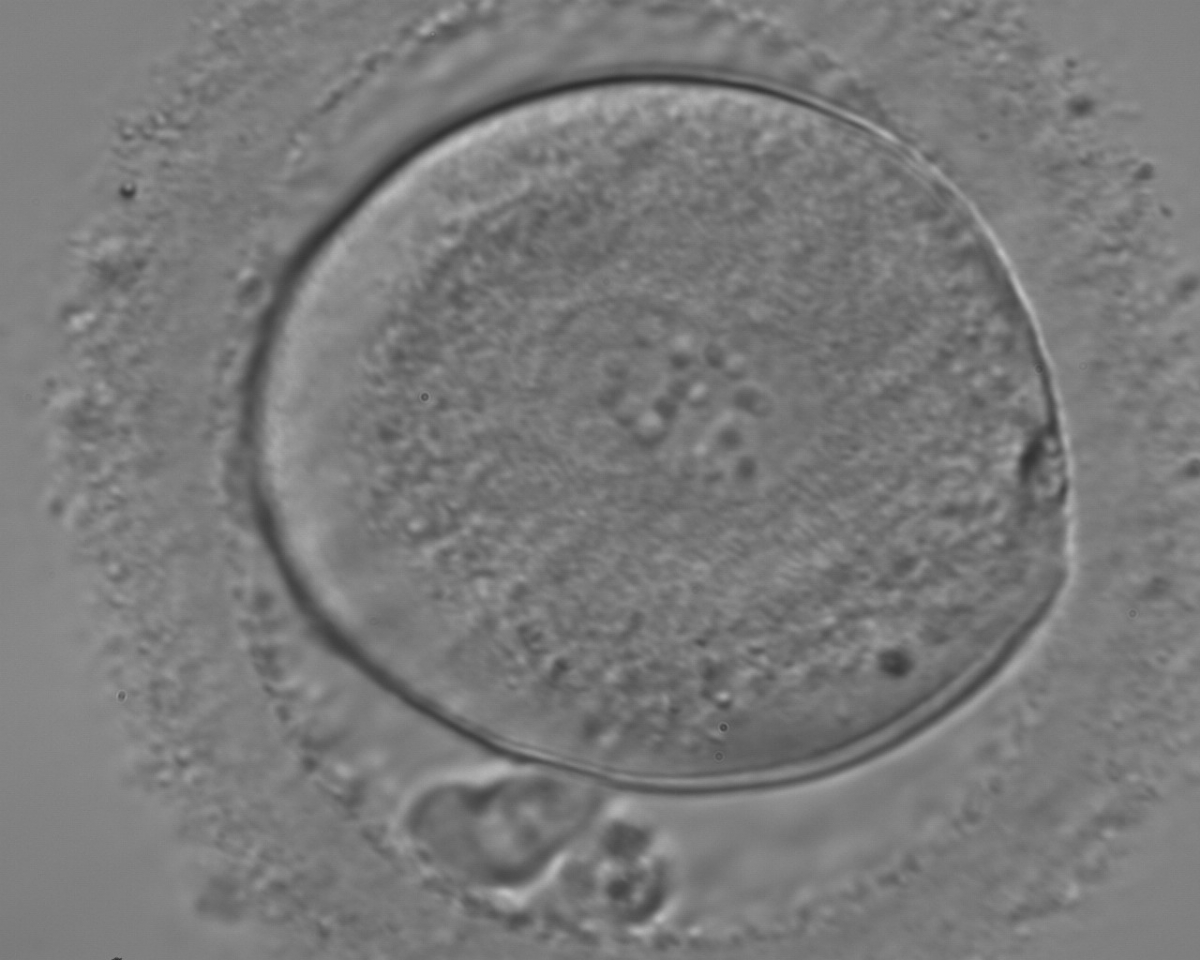
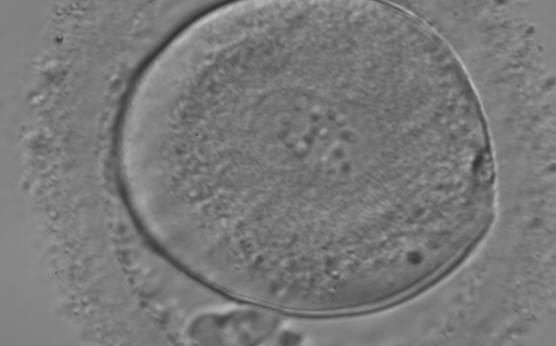
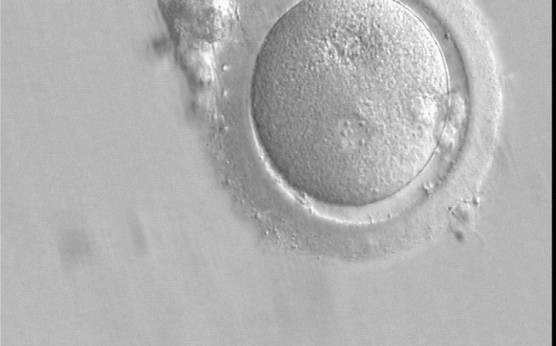
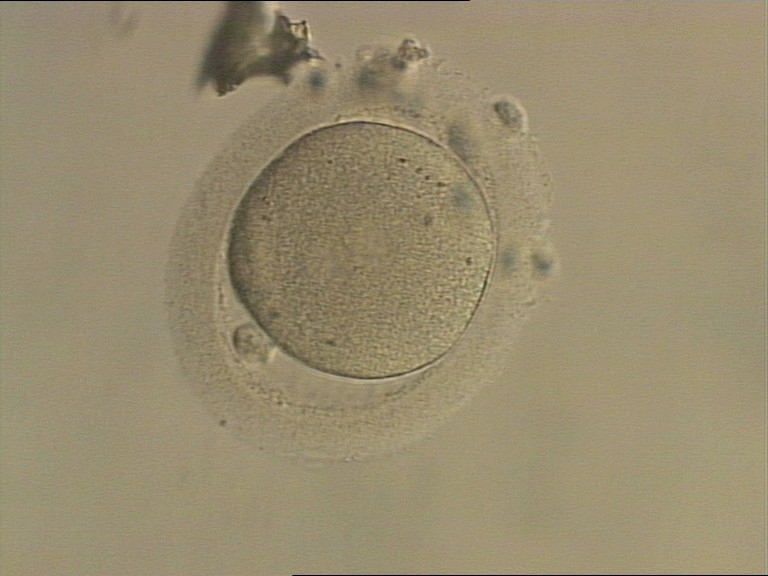
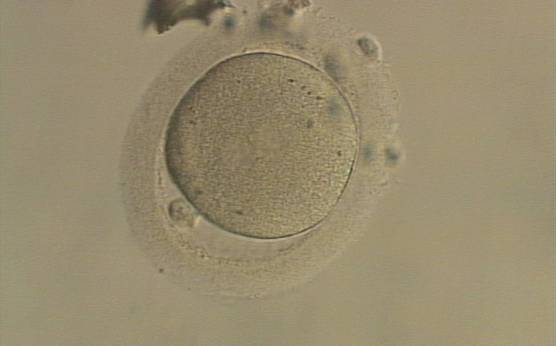
C. Pronuclear morphology
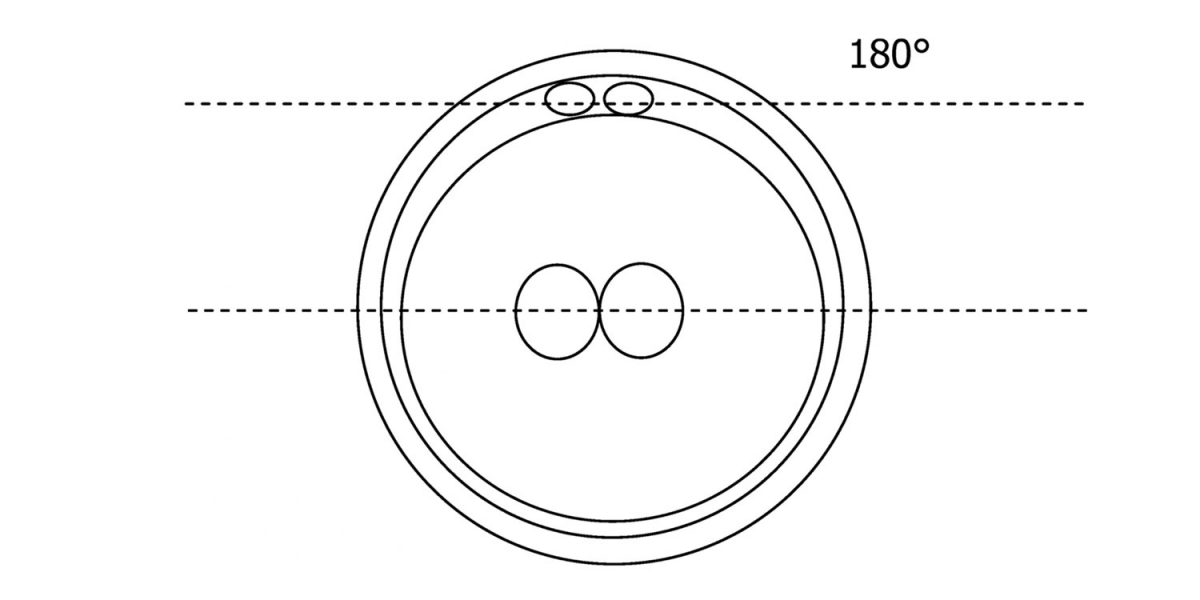
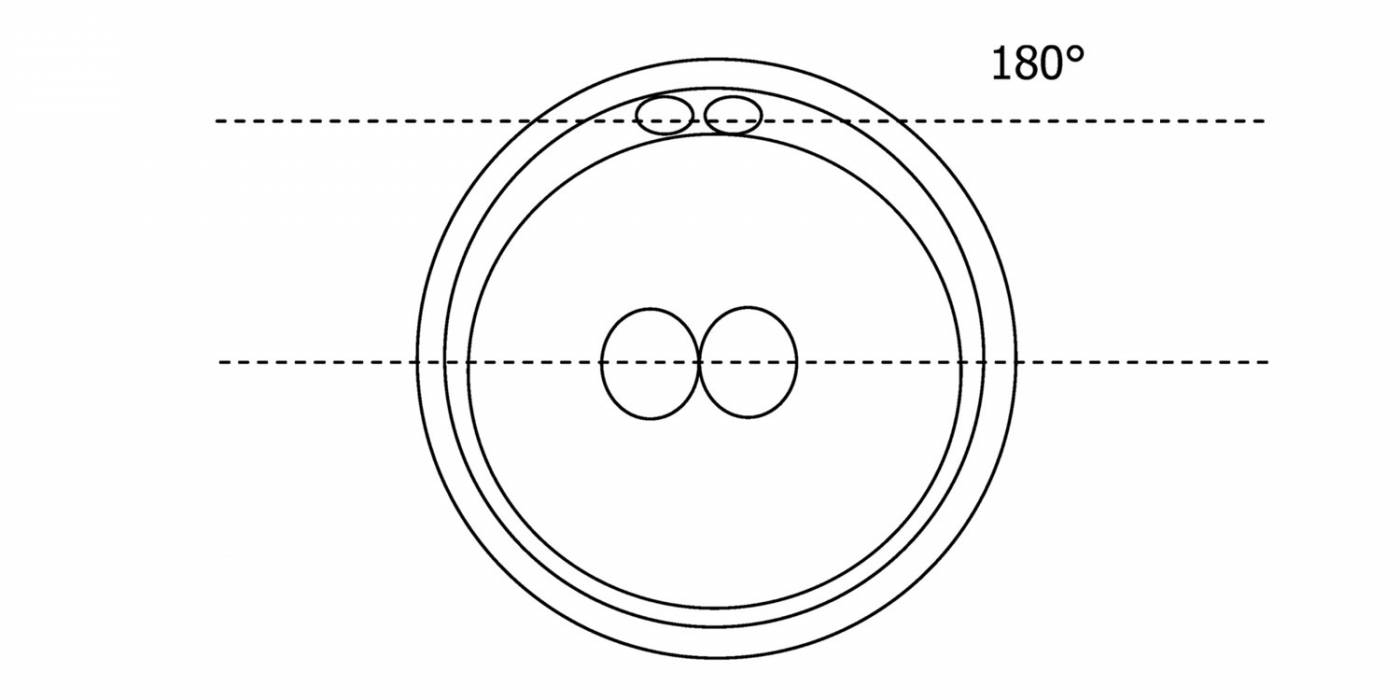
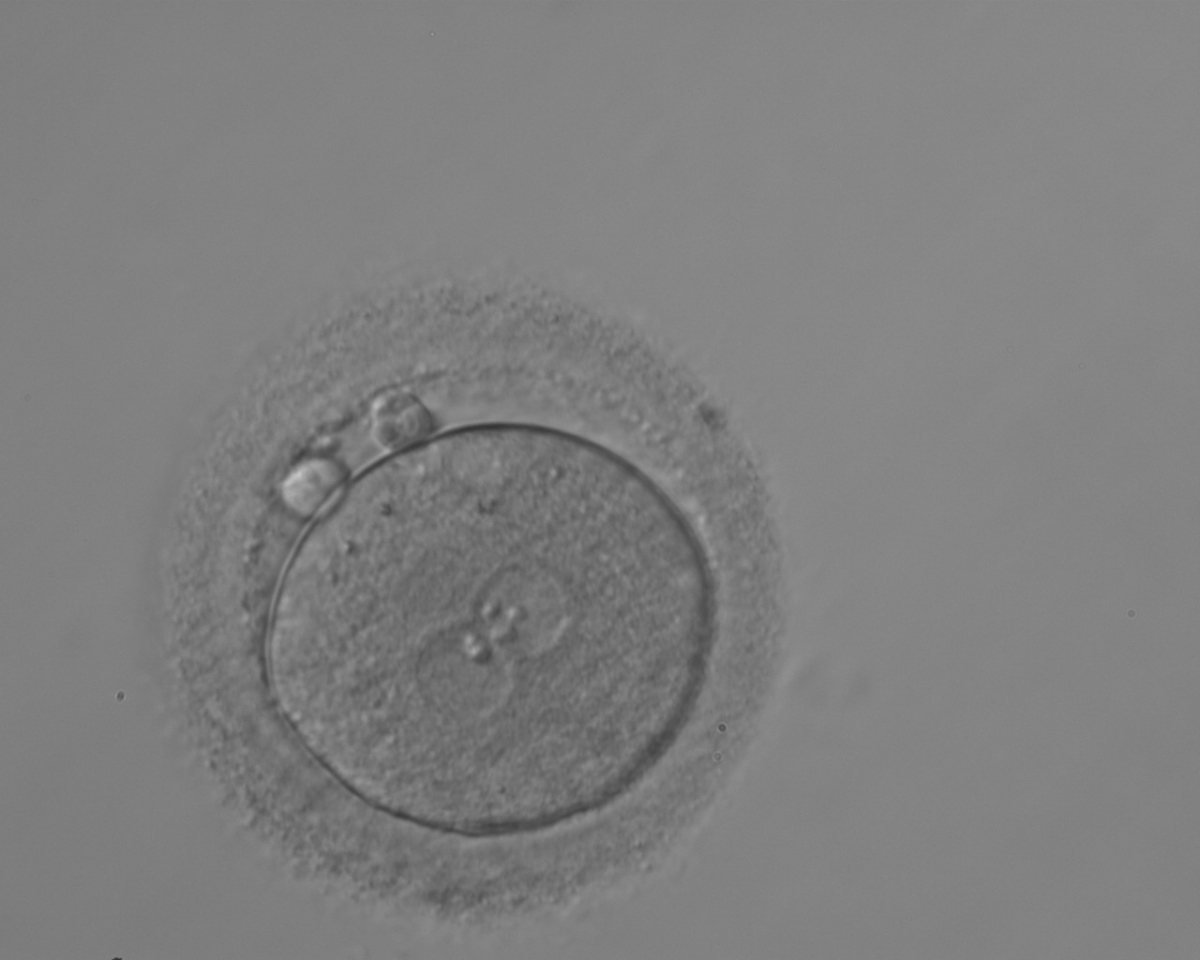
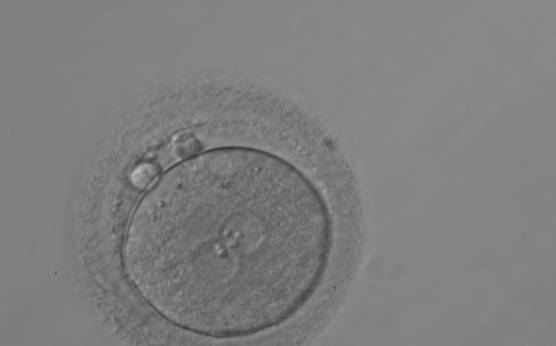
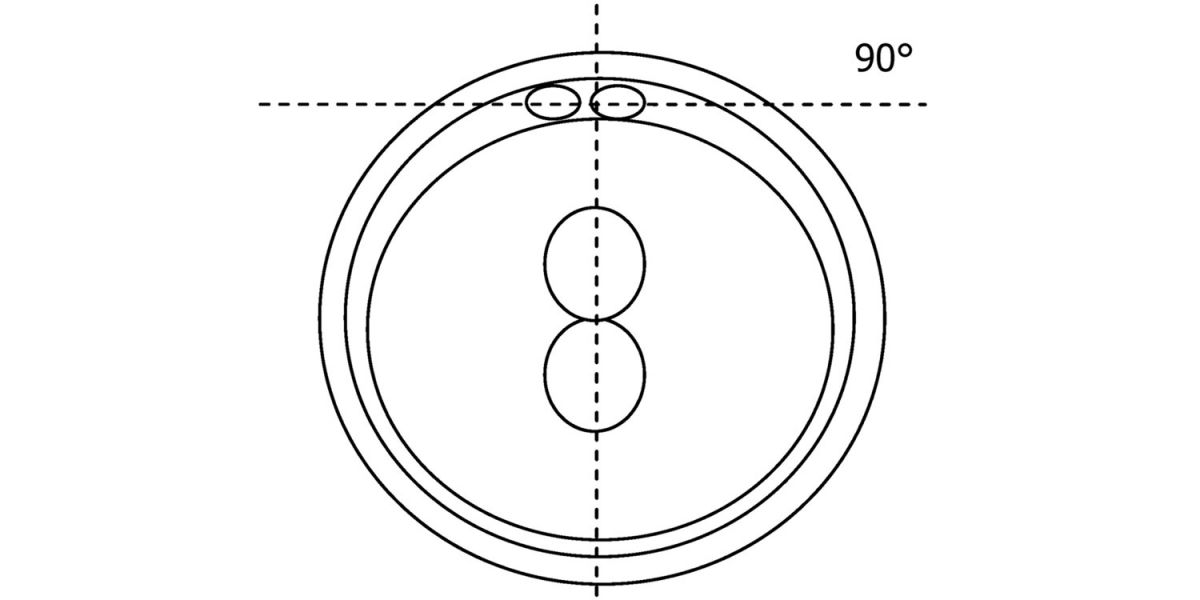
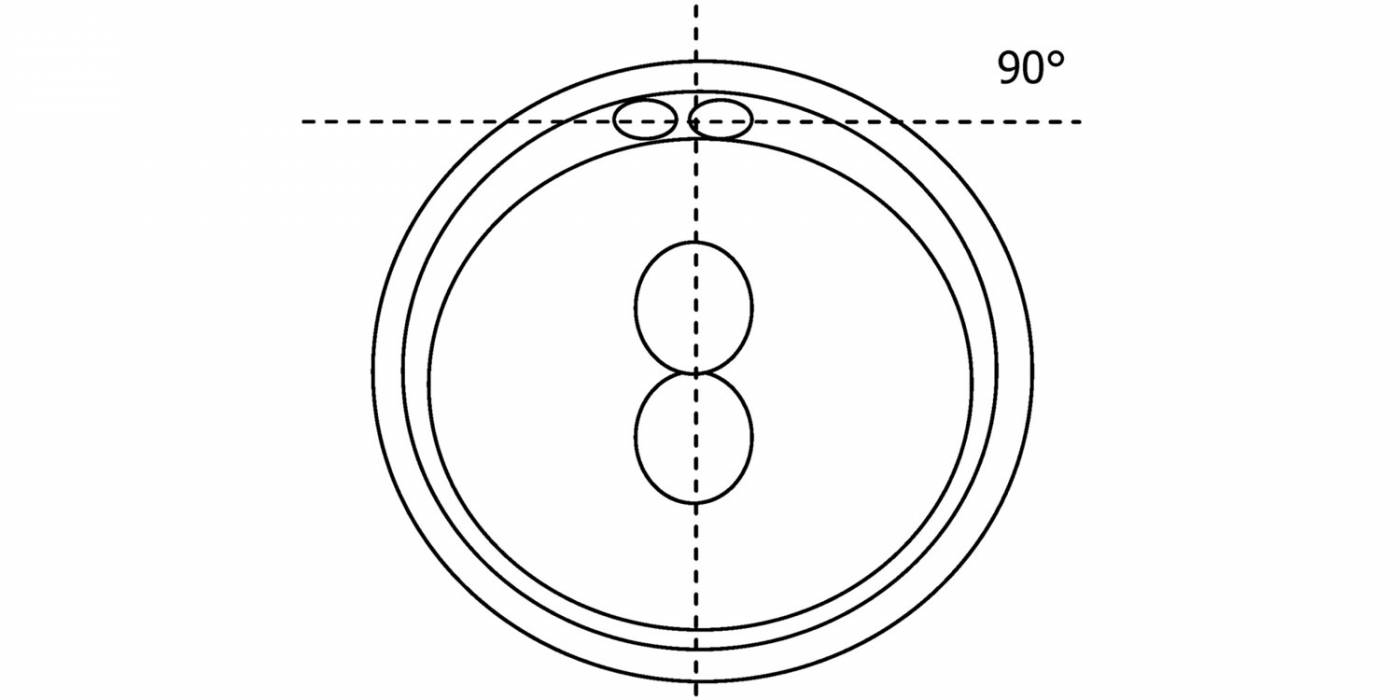
C.1 Alignment parallel/tangential to the plane of the polar bodies
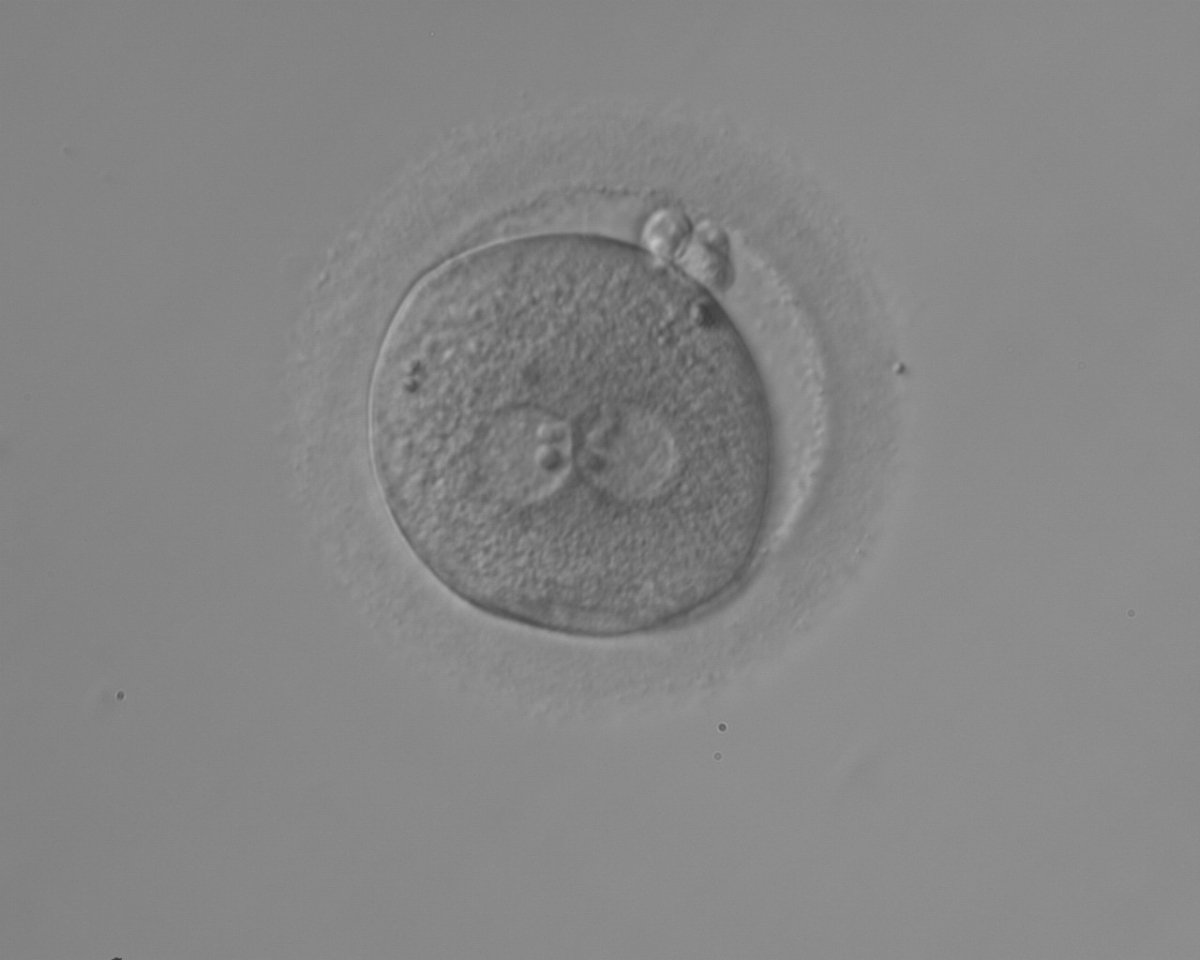
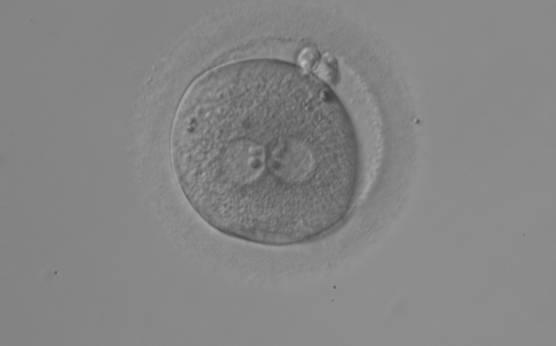
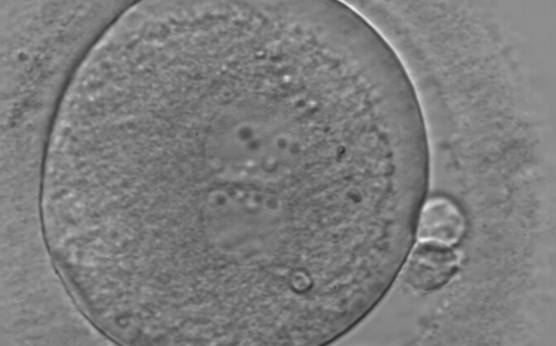
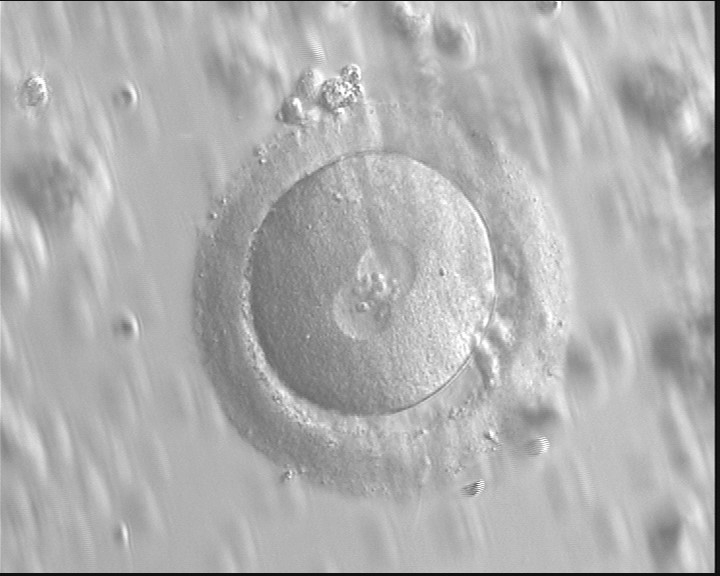
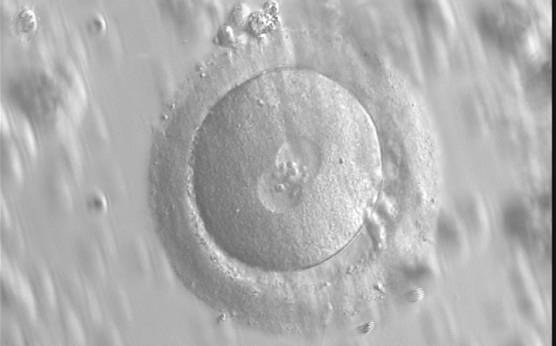
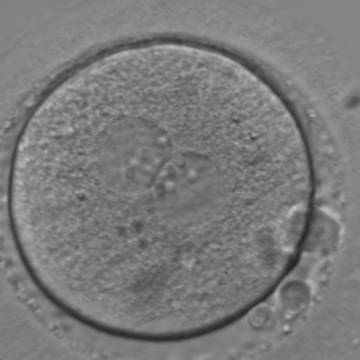
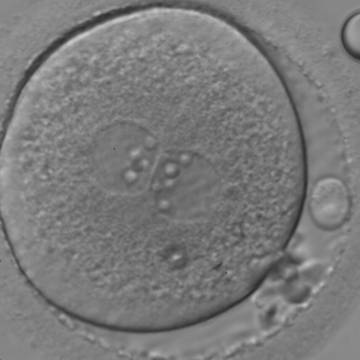
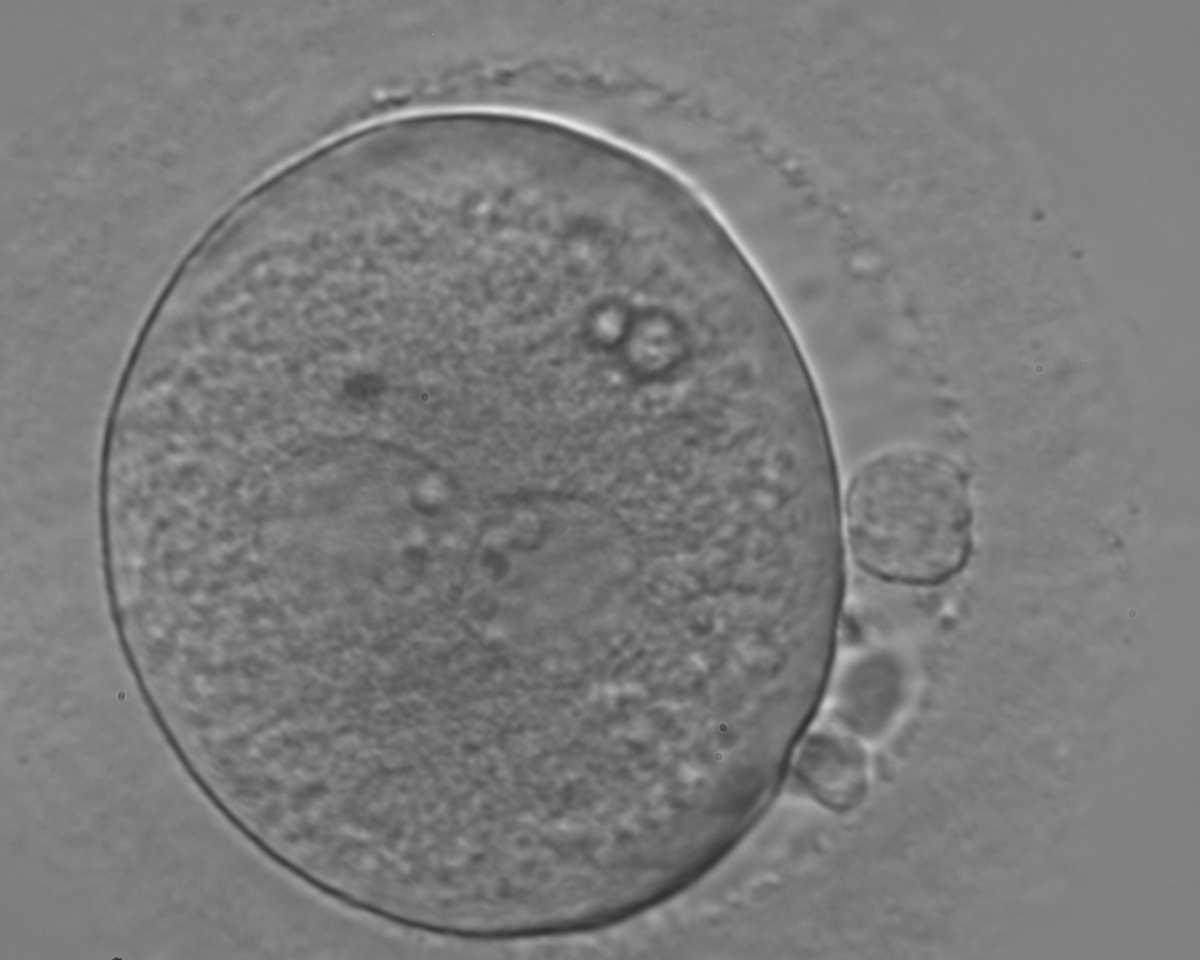
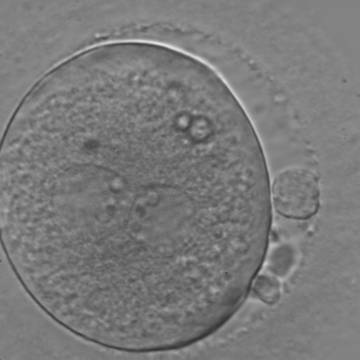
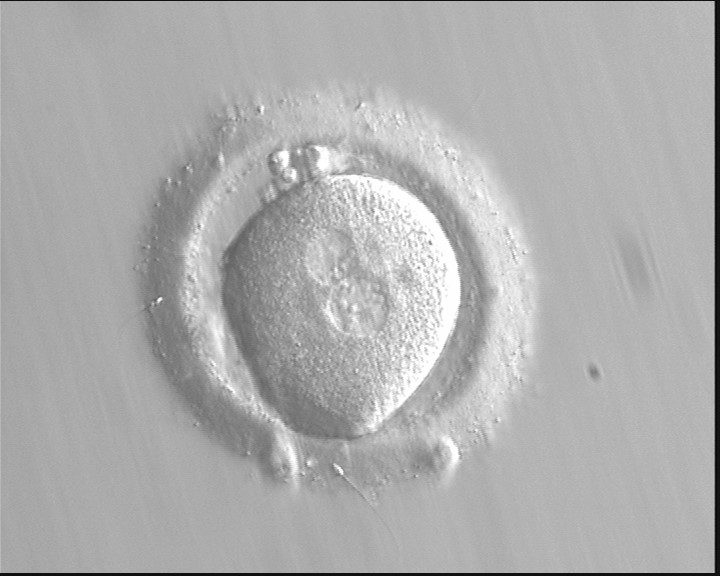
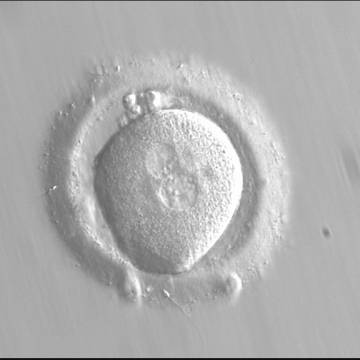
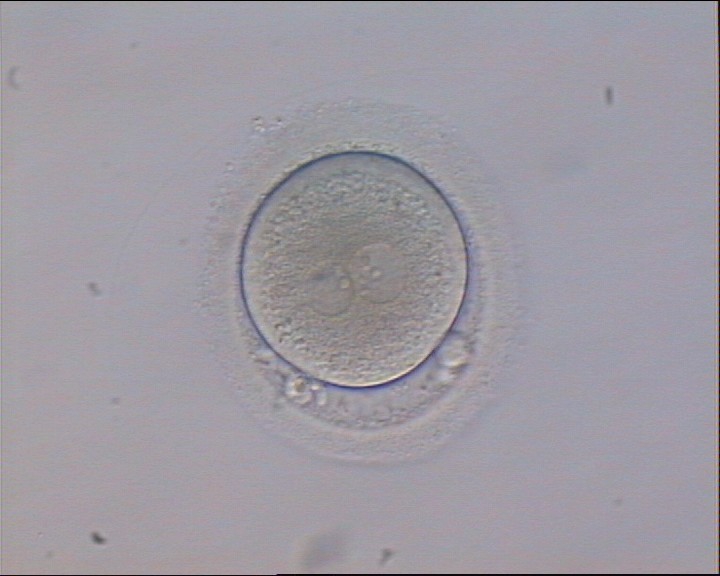
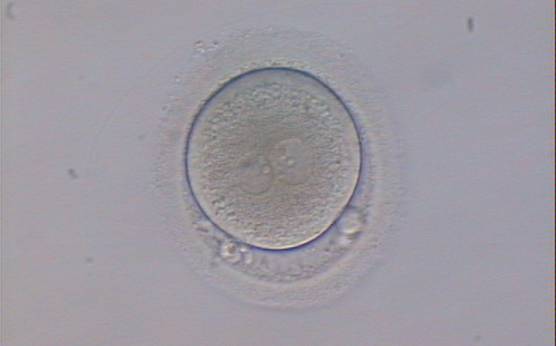
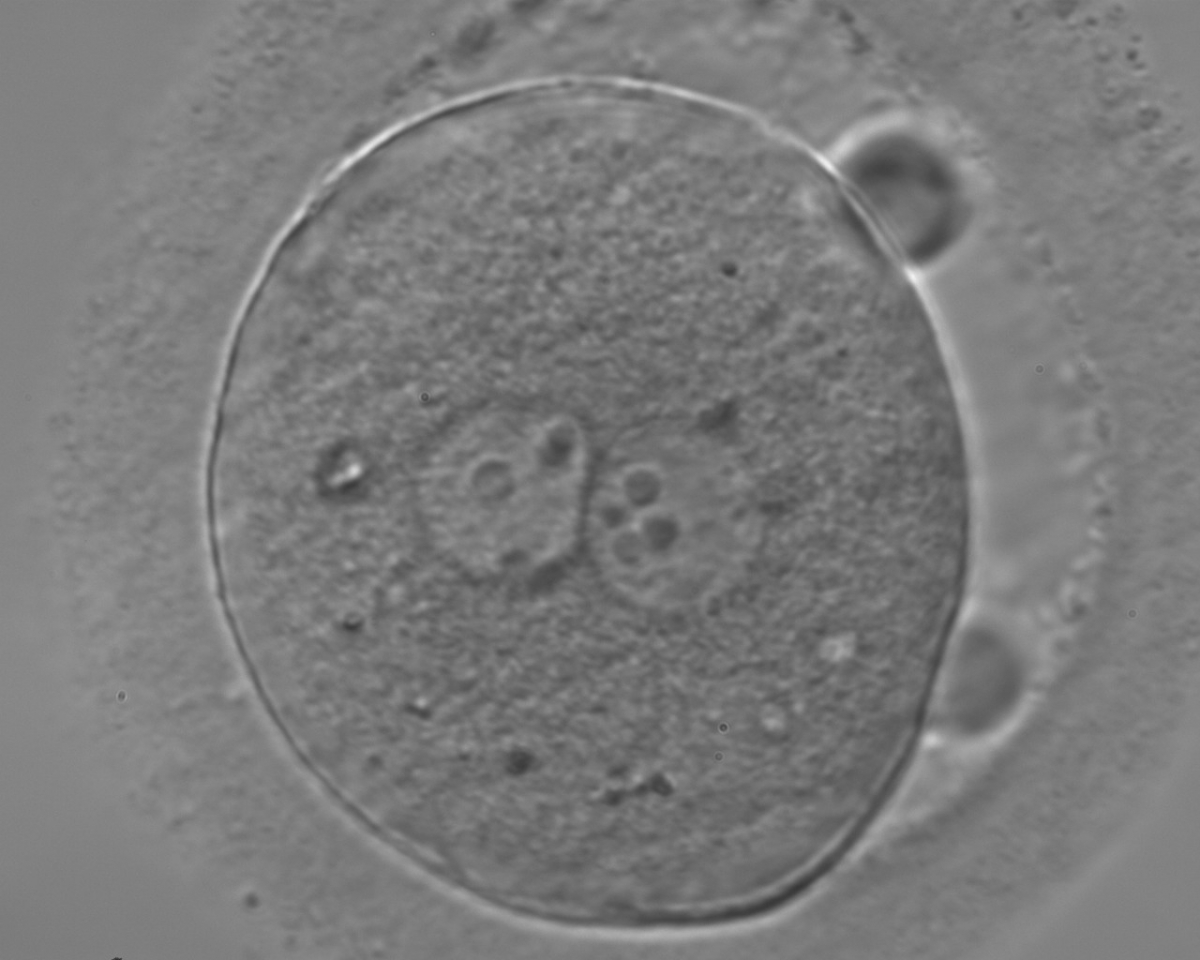
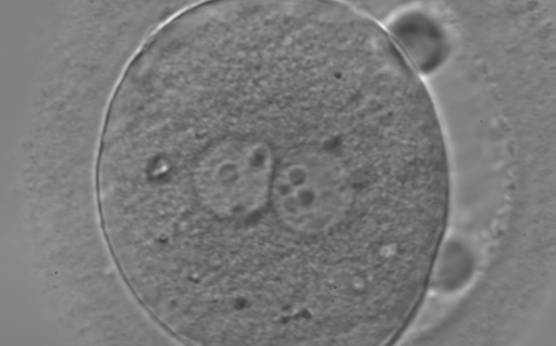
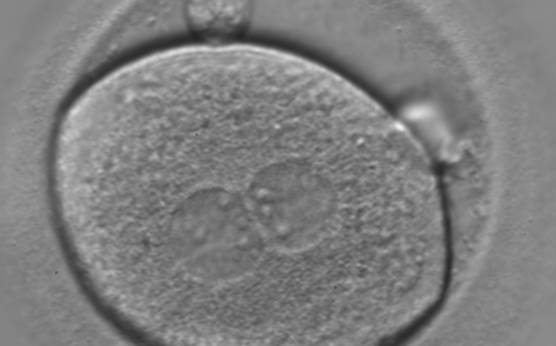
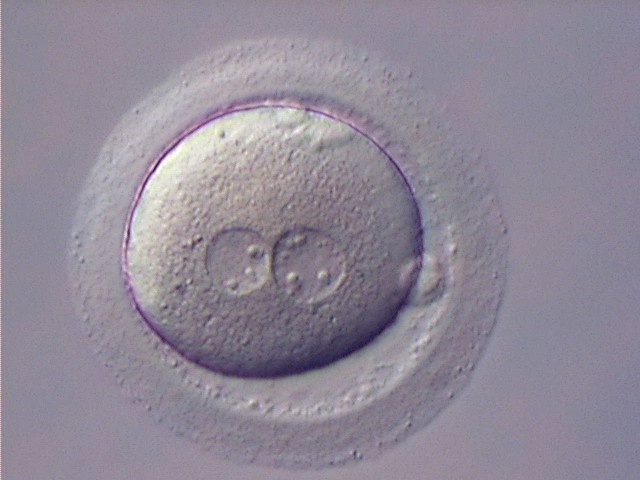
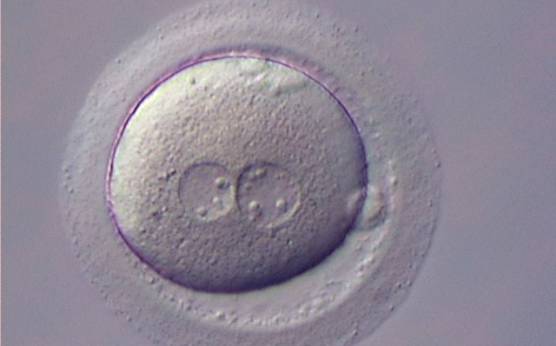
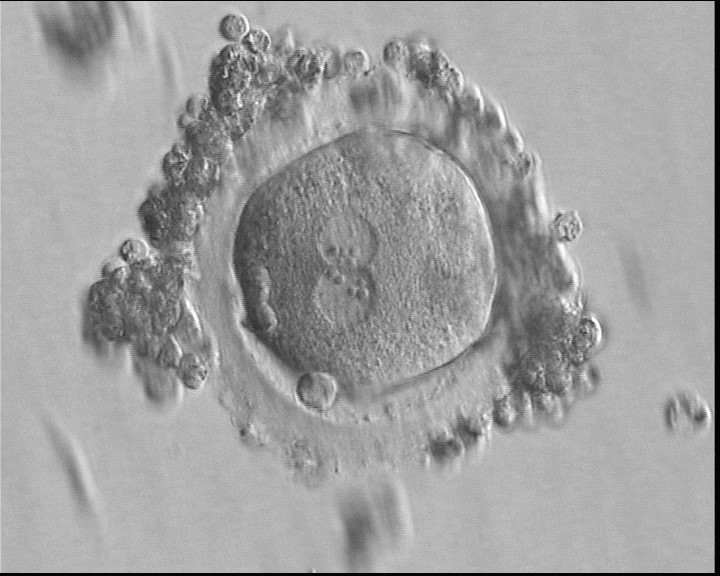
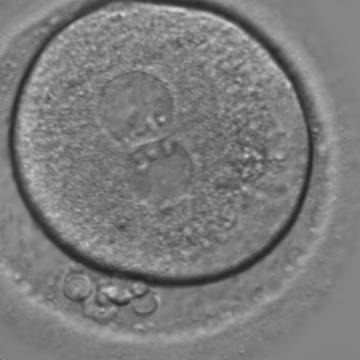
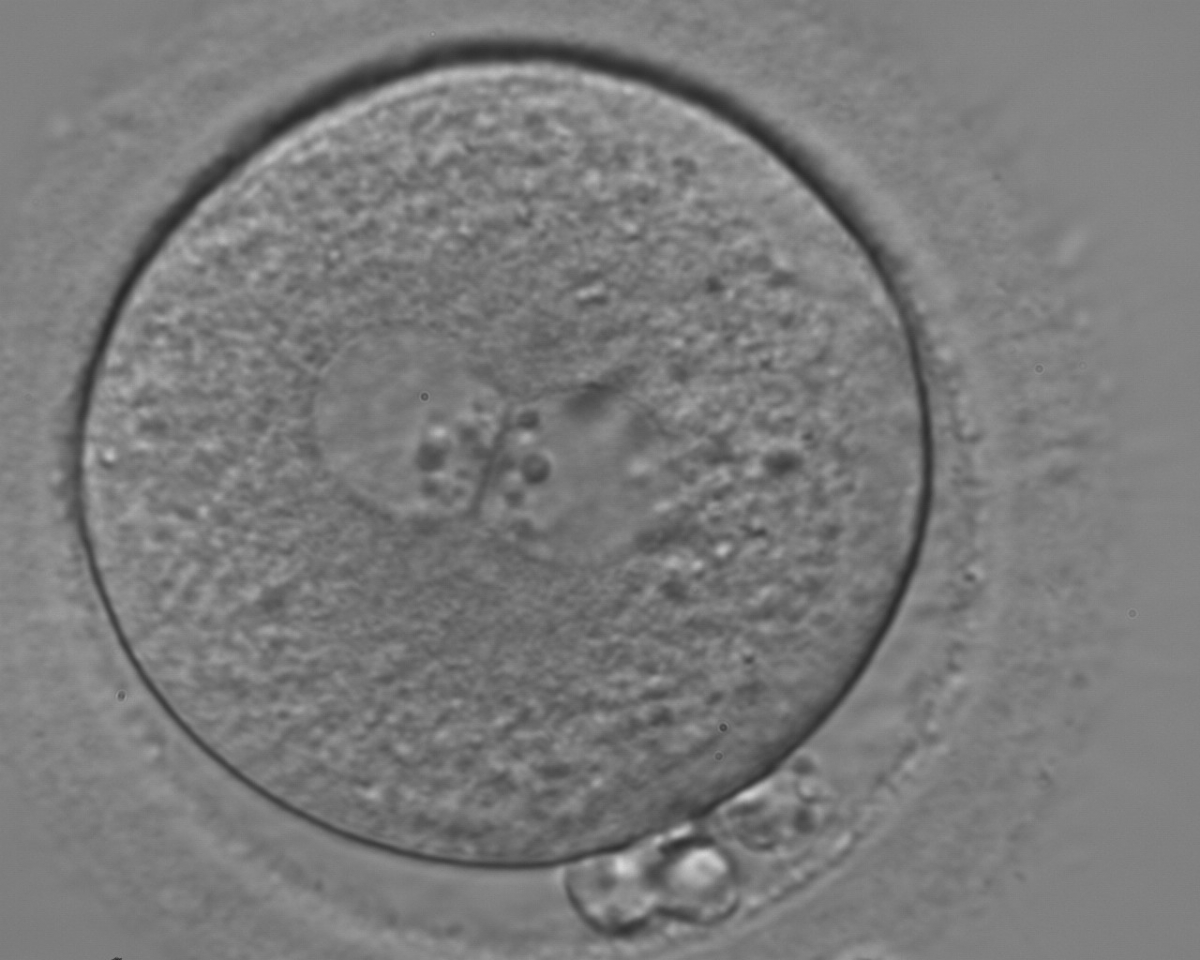
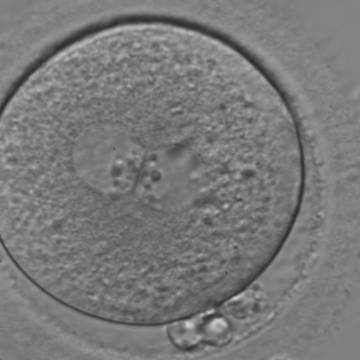
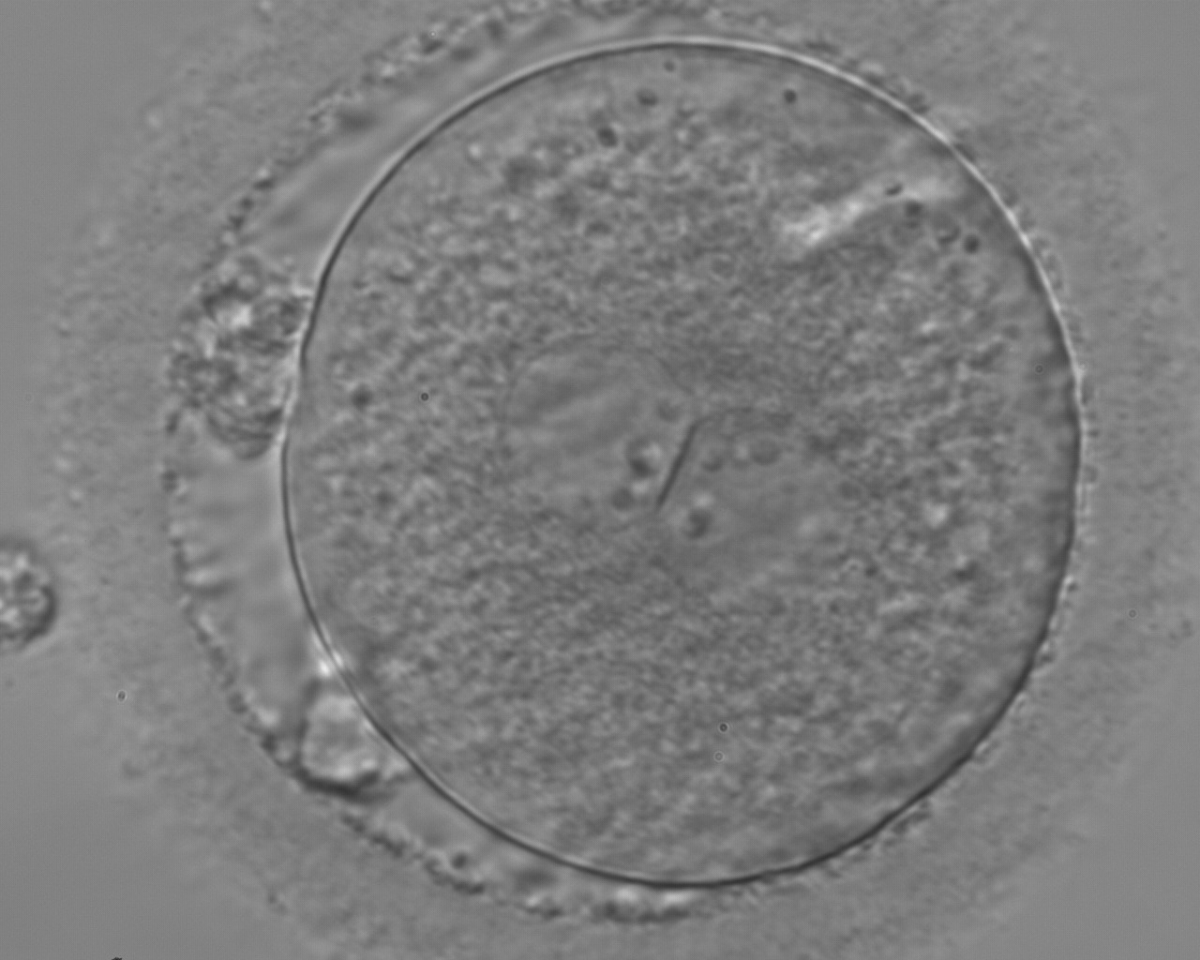
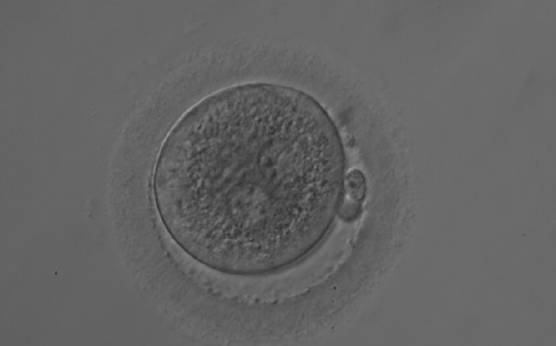
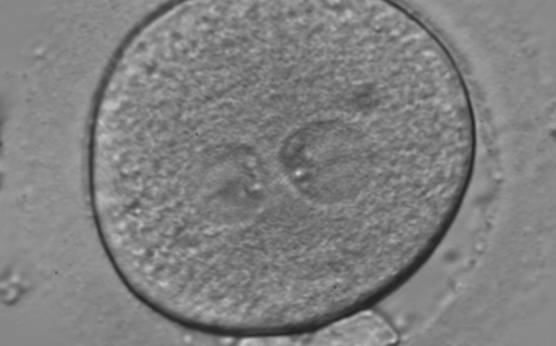
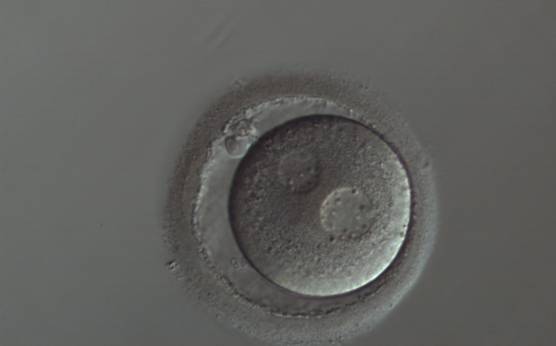
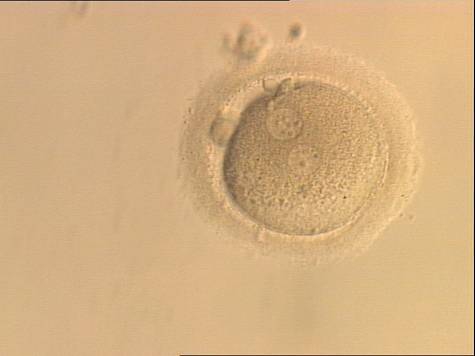
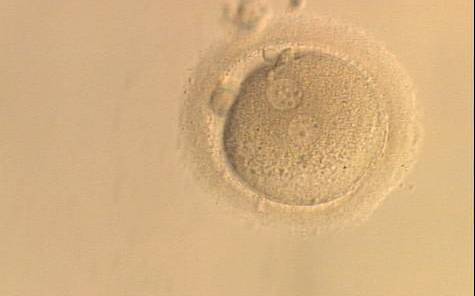
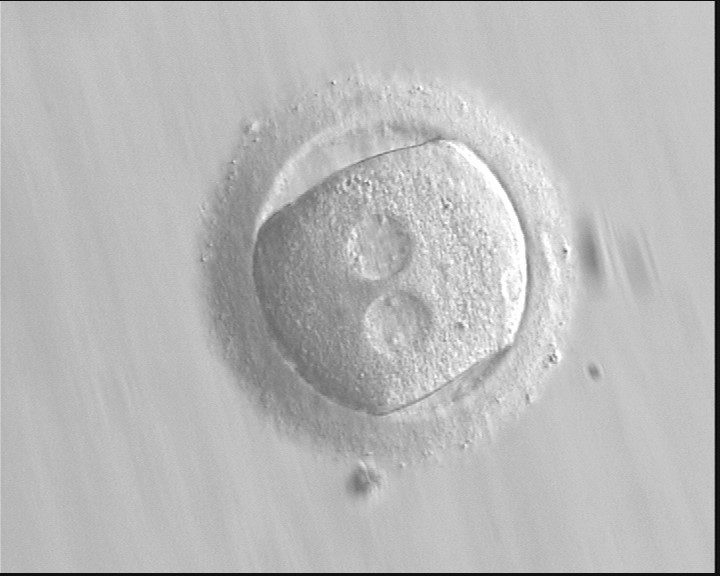
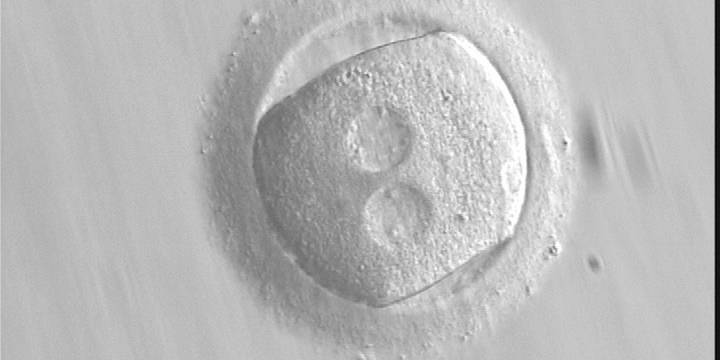
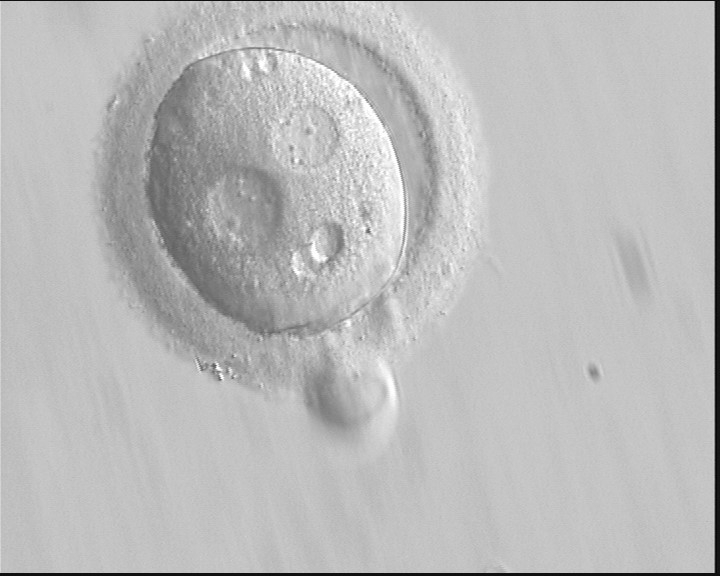
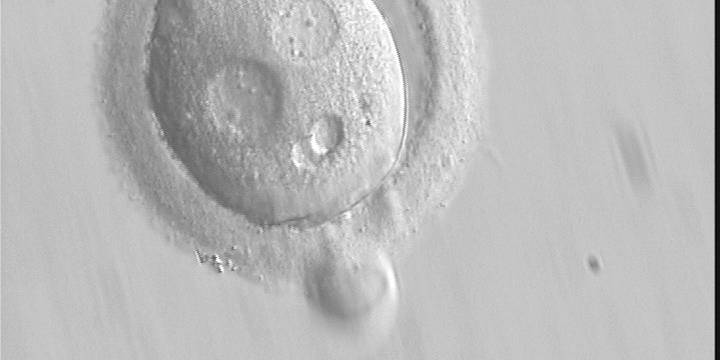
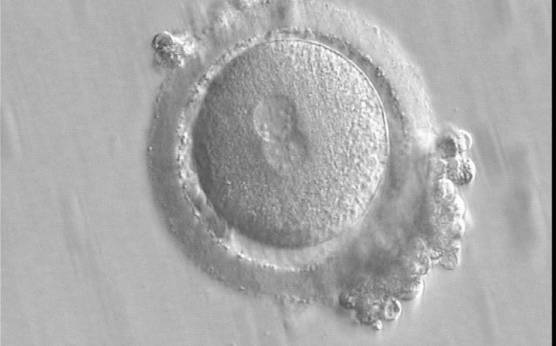
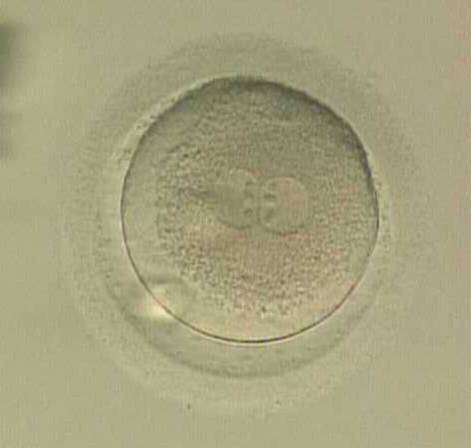
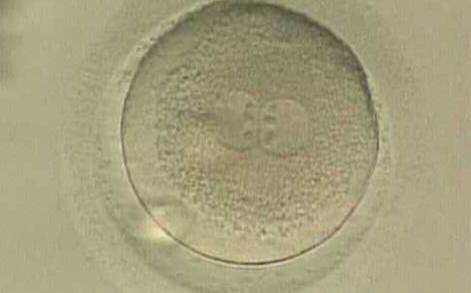
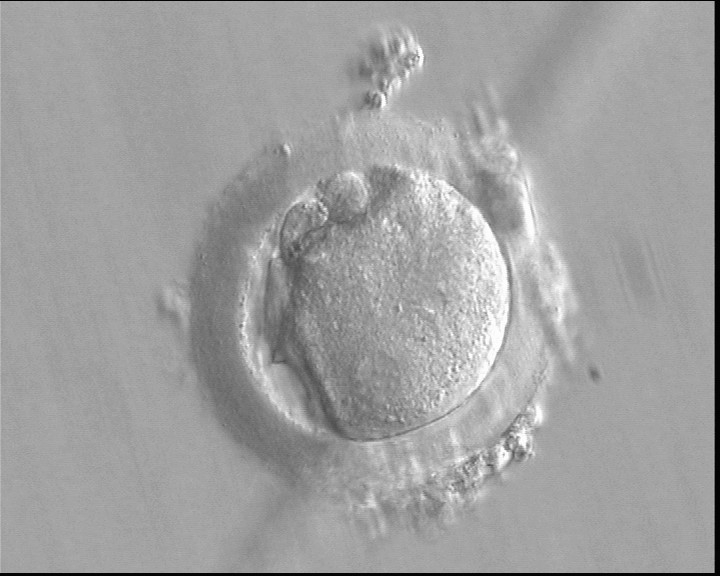
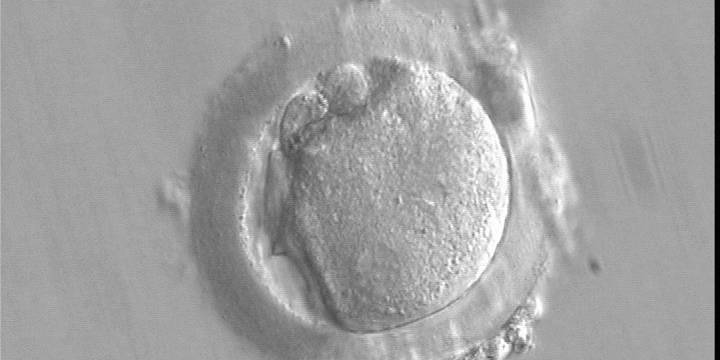
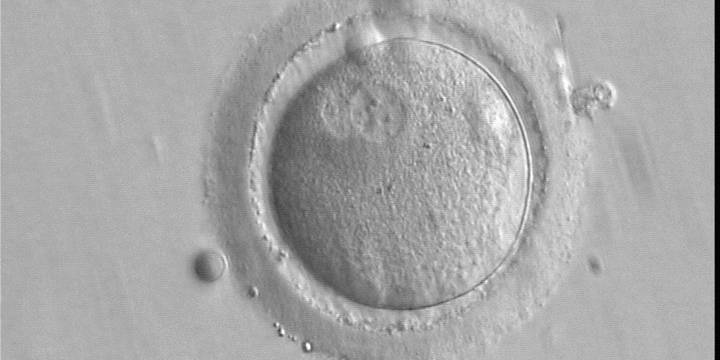
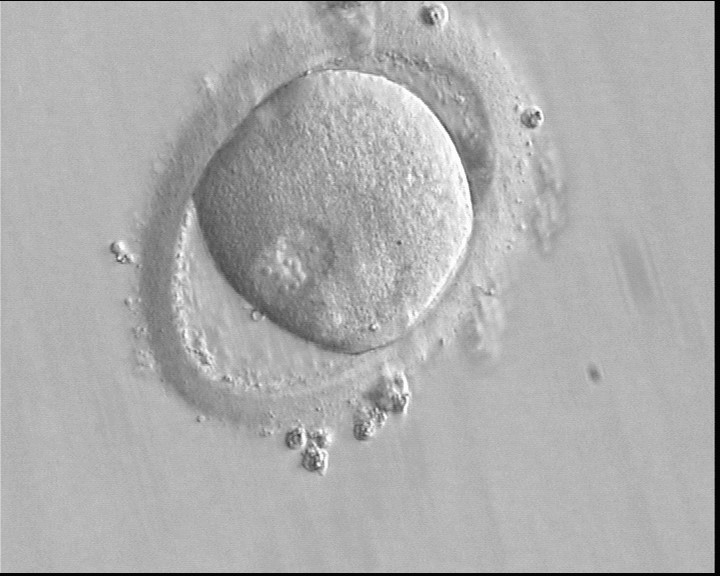
With the extrusion of the second polar body, following sperm entry and oocyte activation, the polar axis is established. Correct alignment of PNs onto this axis is necessary for the formation of polar axes at syngamy and subsequent completion of the first cleavage division and normal development (Edwards and Beard, 1997; Payne et al., 1997; Garello et al., 1999; Gardner, 2001; Scott, 2001). At apposition, the chromatin of both PNs begins to polarize and rotate to face each other with the male PN rotating onto the female PN and placing the centrosome into the furrow between the two PNs (Van Blerkom et al., 1997). In this way, the longitudinal axis of PNs is parallel to the plane of polar bodies (Figs 111–115). Further rotation brings the PNs aligned onto the polar axis; the position of the second polar body defines the plane of the first cleavage division (Figs 116–121). All these movements and rotations could cause the formation of the clear cortical zone known as the halo (Figs 114, 118 and 119).
C.2 Large angle between polar bodies
The failure of PNs to be juxtaposed and centrally positioned within the cytoplasm or having non-aligned polar bodies with respect to PNs at fertilization check could result in altered development, i.e. fertilization failure and abnormal cleavage of the embryo (Gianaroli et al., 2003; Alpha Scientists in Reproductive medicine and ESHRE Special Interest Group of Embryology, 2011). Large angles between polar bodies (Figs 122–124) have been suggested to be predictors of poor embryo development (Gianaroli et al., 2003). This effect could be due to sub-optimal orientation of PNs (Figs 125 and 126) generating a great degree of cytoplasmic turbulence, which could facilitate uneven cleavage or fragmentation (Garello et al., 1999).
C.3 Abutment/separation between PNs
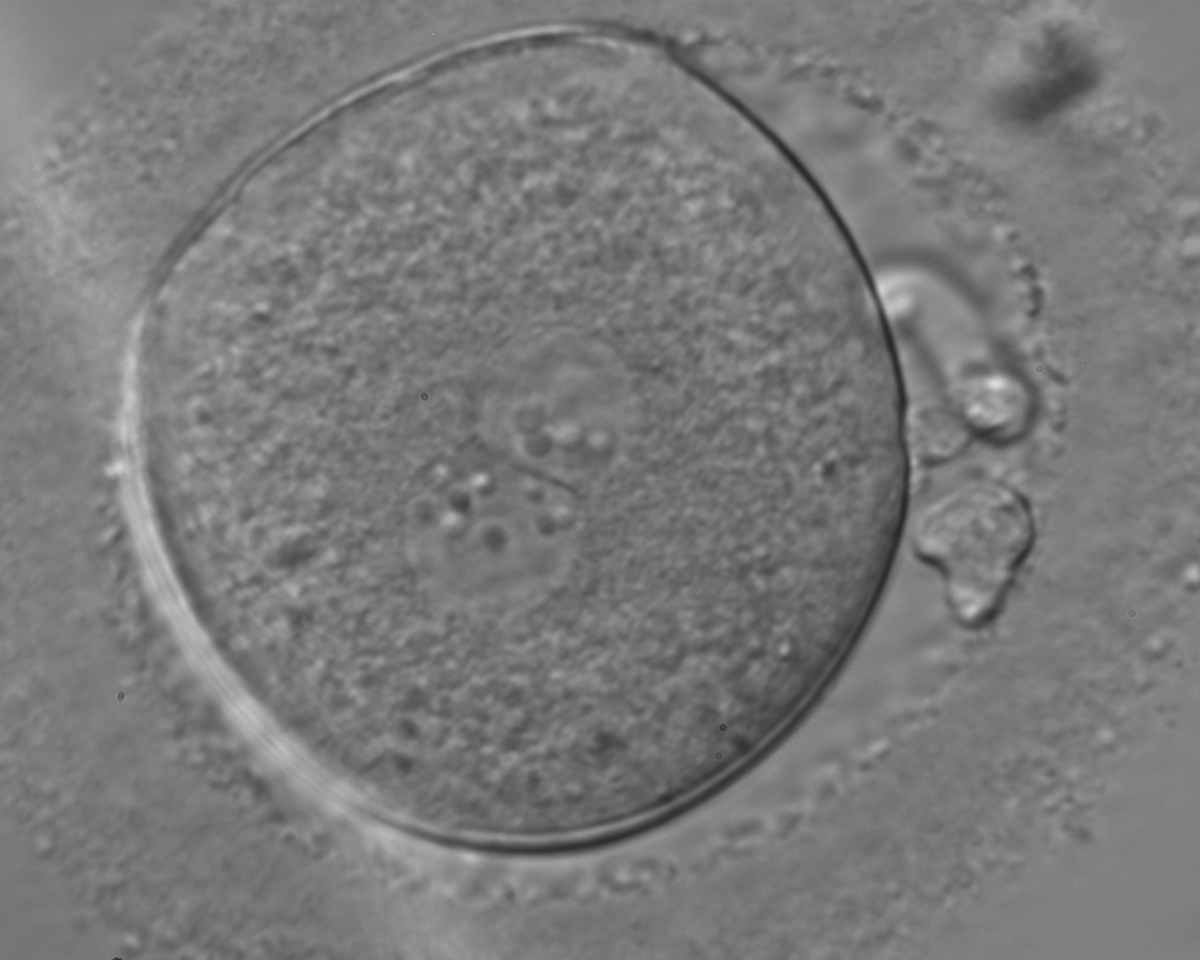
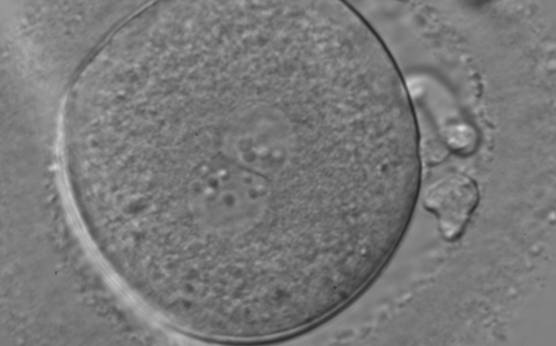
In human oocytes, the aster from the sperm centrosome organizes the microtubules, which control the abutment and apposition of PNs (Figs 127–131), and direct the formation of polar axes at syngamy by setting the plane of the first division. The subsequent movements and rotations favour the distribution of the mitochondria and chromatin alignment, which are essential for correct development.
Failed progression to apposition and syngamy (Figs 132–134) mostly depends on sperm centrosome activity. Observations of zygotes with separated PNs at fertilization check during subsequent development (Figs 135–136) demonstrate severe delay or arrest in development (Fig. 136) in almost 80% of cases (Gianaroli et al., 2003). This condition is most frequently associated with pathological spermatozoa, particularly from epididymal or testicular samples (Fig. 133).
C.4 Centrally/peripherally positioned PNs
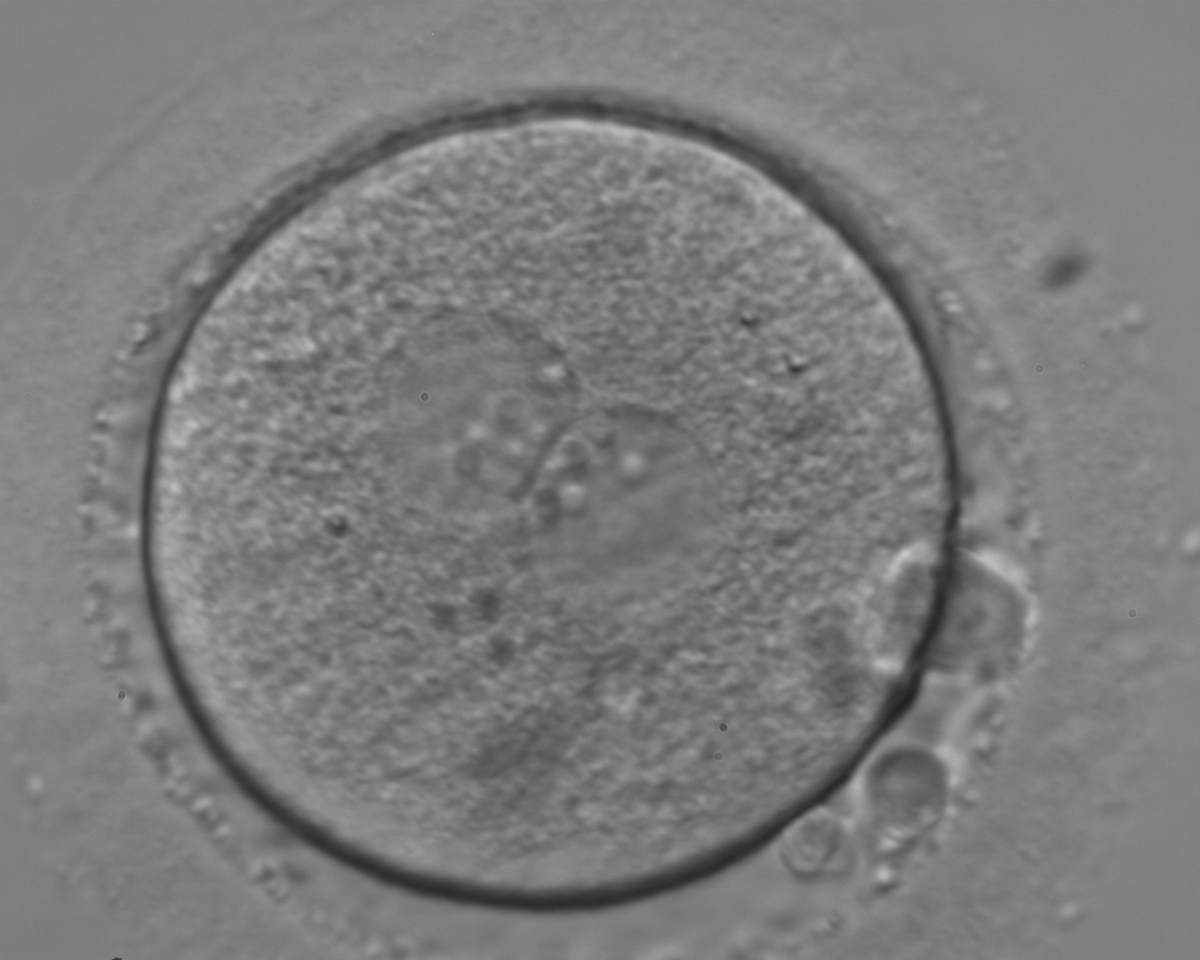
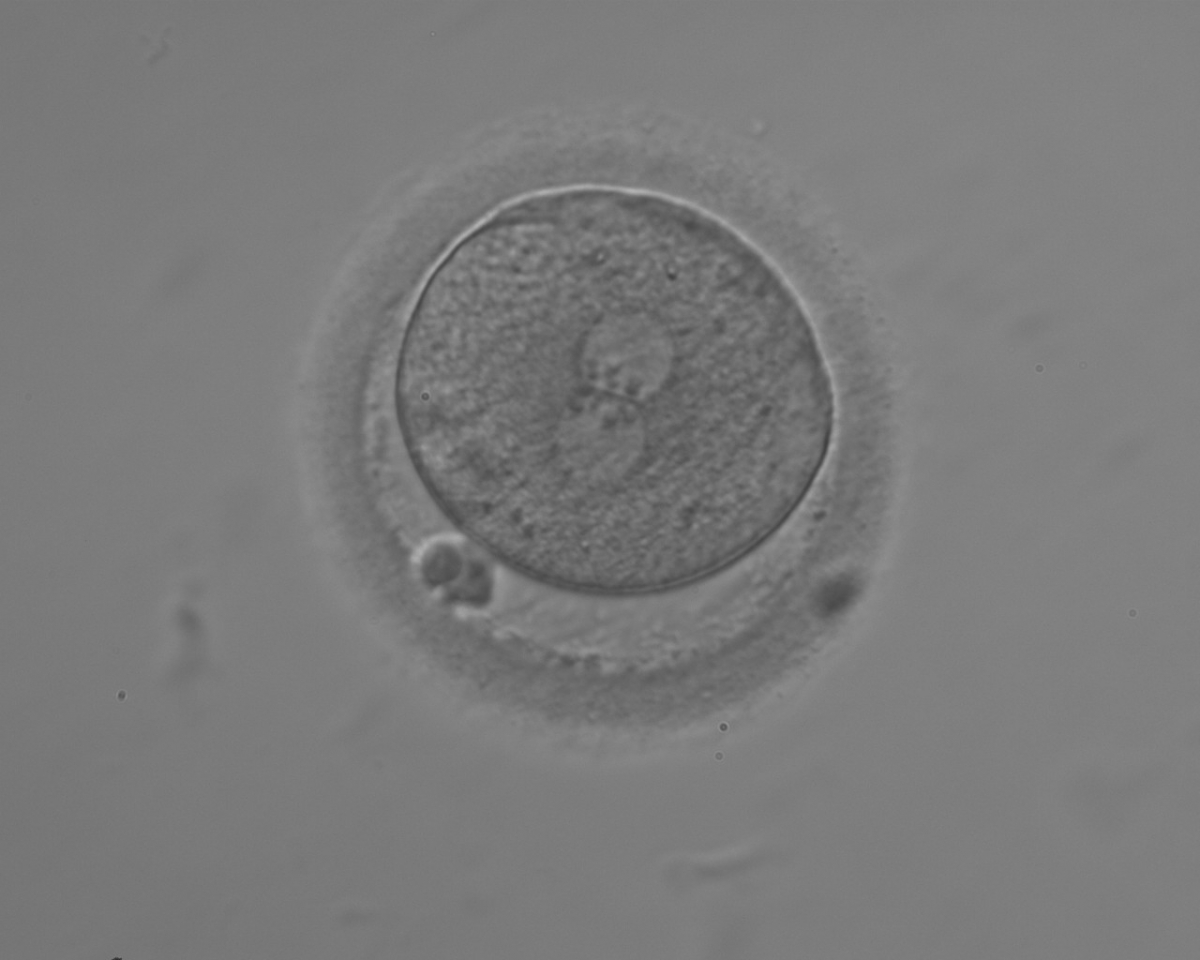
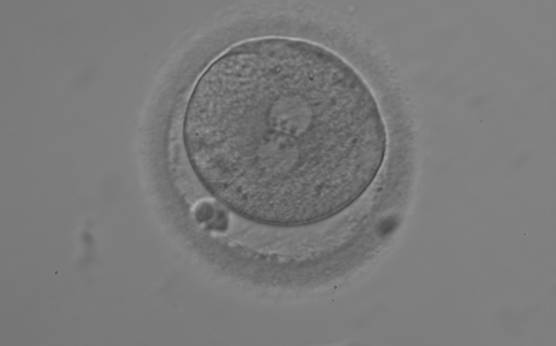
The position of PNs has a relevant effect on the first cleavage plane that normally goes through the pronuclear axis (Scott, 2003). In the majority of zygotes with centrally positioned PNs (Fig. 137), the first cleavage occurs regularly, giving rise to normally developing embryos. Due to the dynamics of PN movements within the cytoplasm, their orientation on the polar axis varies depending on the progression of rotation (Figs 138 and 139) towards the final state which determines the first cleavage plane (Fig. 140).
In cases of peripherally positioned PNs (Figs 141 and 142), cleavage occurs according to the pronuclear axis and results frequently in abnormal morphology (Fig. 143), cleavage (Fig. 144) and arrest of development (Fig. 145). Nevertheless implantation can occur (Fig. 146).
C.5 Pronuclear membrane breakdown/syngamy
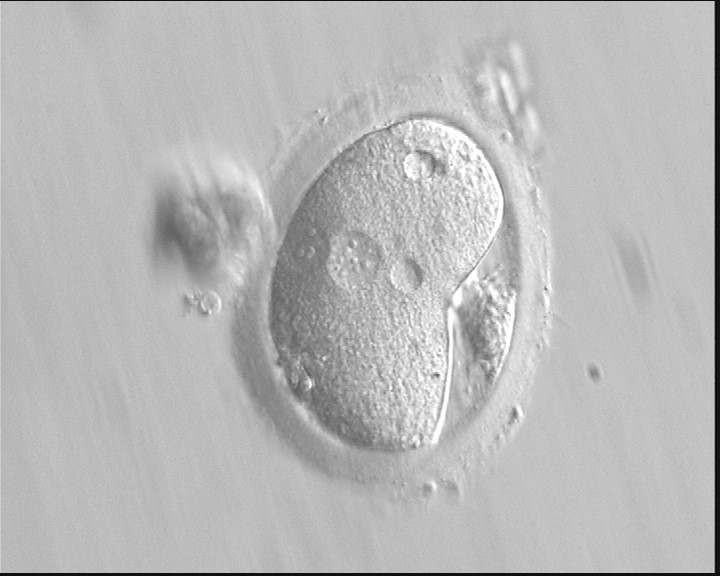
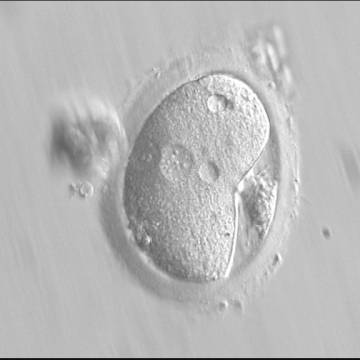
Following sperm entry, both PNs migrate toward each other while replicating their own DNA. At juxtaposition, nuclear membranes break down a few hours prior to initiation of the first cleavage division (Figs 147 and 148). Syngamy occurs (Fig. 149) and the two sets of haploid genomes merge.
The astral centrosome containing two centrioles splits and relocates to opposite poles of a bipolar spindle to establish bipolarization that controls cell division. The centrioles take a pivotal position on spindle poles, while chromosomes organize on the equator of the metaphase plate. Anaphase and telophase ensue completing the first mitotic division.
In contrast to some animal species, membrane fusion of PNs (Fig. 150) is not a common process in human zygotes.
Article references:
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 2011;26:1270-1283.
Abstract/FREE Full Text
Edwards RG, Beard HK. Oocyte polarity and cell determination in early mammalian embryos. Mol Hum Reprod 1997;3:863-905.
Abstract/FREE Full Text
Gardner RL. Specification of embryonic axes begins before cleavage in normal mouse development. Development 2001;128:839-847.
Abstract
Garello C, Baker H, Rai J, Montgomery S, Wilson P, Kennedy CR, Hartshorne GM. Pronuclear orientation, polar body placement, and embryo quality after intracytoplasmic sperm injection and in vitro fertilization: further evidence for polarity in human oocytes? Hum Reprod 1999;14:2588-2595.
Abstract/FREE Full Text
Gianaroli L, Magli MC, Ferraretti AP, Fortini D, Grieco N. Pronuclear morphology and chromosomal abnormalities as scoring criteria for embryo selection. Fertil Steril 2003;80:341-349.
CrossRef | Medline | Web of Science | Google Scholar
Montag M, Liebenthron J, Köster M. Which morphological scoring system is relevant in human embryo development? Placenta 2011;32:S252-S256.
CrossRef | Medline | Web of Science | Google Scholar
Payne D, Flaherty SP, Barry MF, Matthews CD. Preliminary observations on polar body extrusion and pronuclear formation in human oocytes using time-lapse video cinematography. Hum Reprod 1997;12:532-541.
Abstract/FREE Full Text
Payne JF, Raburn DJ, Couchman GM, Price TM, Jamison MG, Walmer DK. Relationship between pre-embryo pronuclear morphology (zygote score) and standard day 2 or 3 embryo morphology with regard to assisted reproductive technique outcomes. Fertil Steril 2005;84:900-909.
CrossRef | Medline | Web of Science | Google Scholar
Scott L. Oocyte and embryo polarity. Semin Reprod Med 2001;18:171-183.
Google Scholar
Scott L. Pronuclear scoring as a predictor of embryo development. Reprod Biomed Online 2003;6:201-214.
CrossRef | Medline | Google Scholar
Senn A, Urner F, Chanson A, Primi MP, Wirthner DW, Germond M. Morphological scoring of human pronuclear zygotes for prediction of pregnancy outcome. Hum Reprod 2006;21:234-239.
Abstract/FREE Full Text