B. Inner cell mass morphology
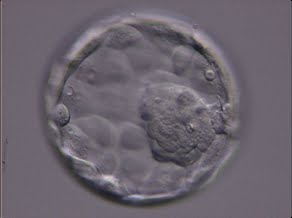
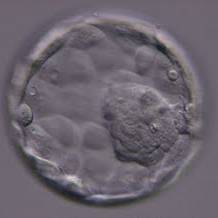
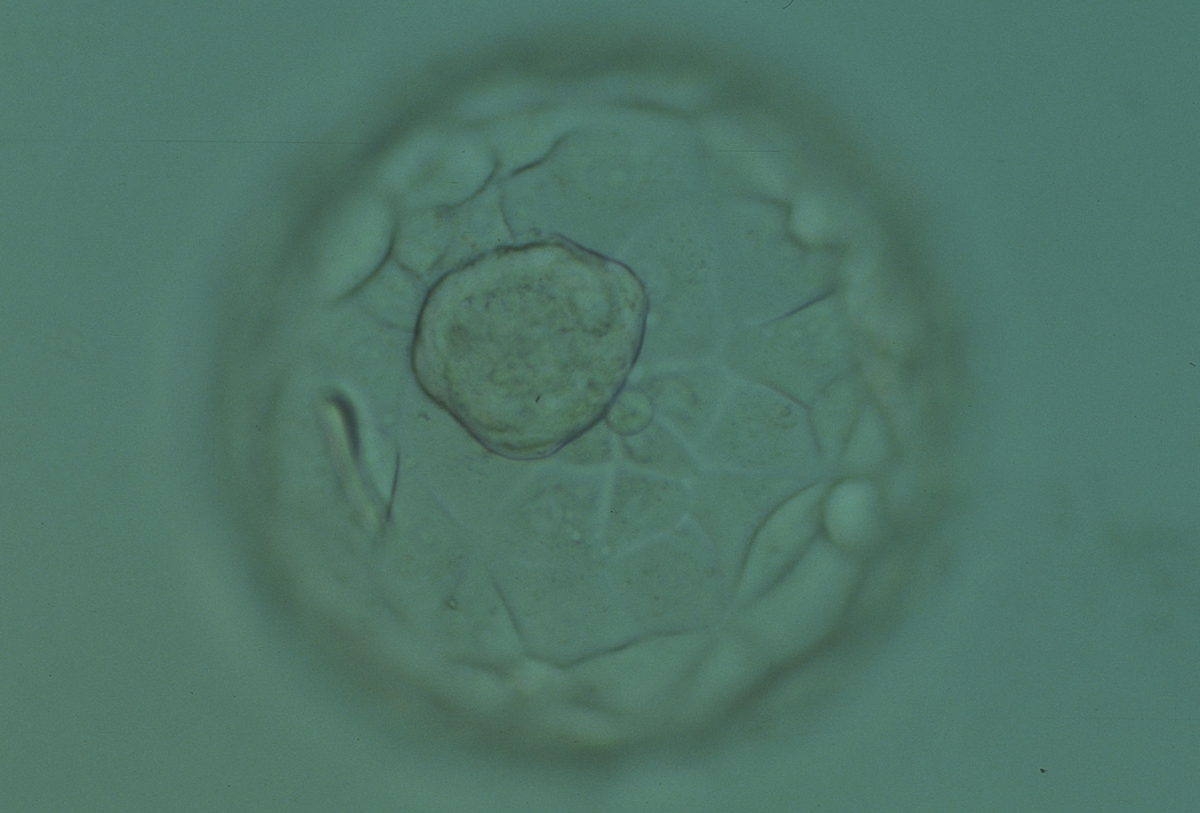
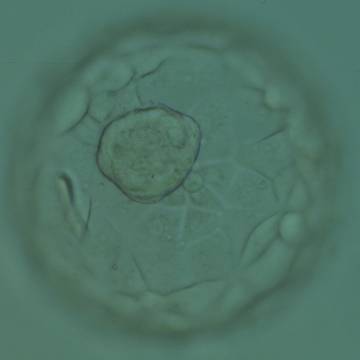
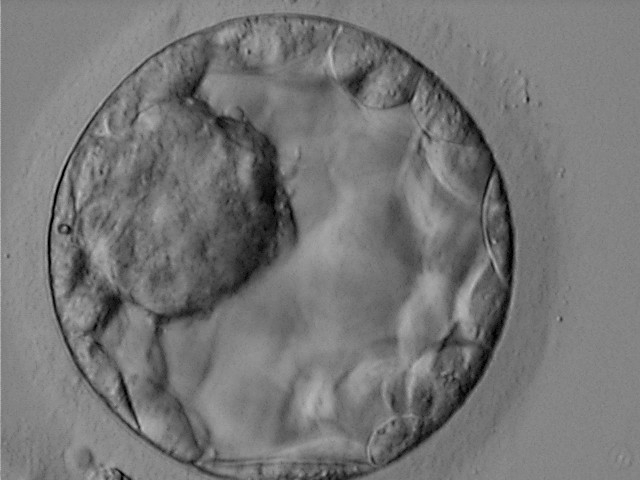
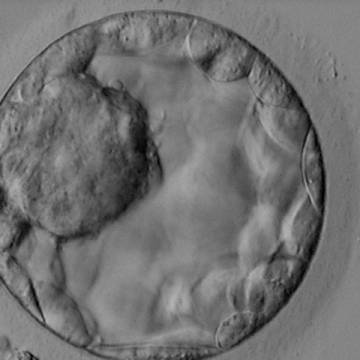
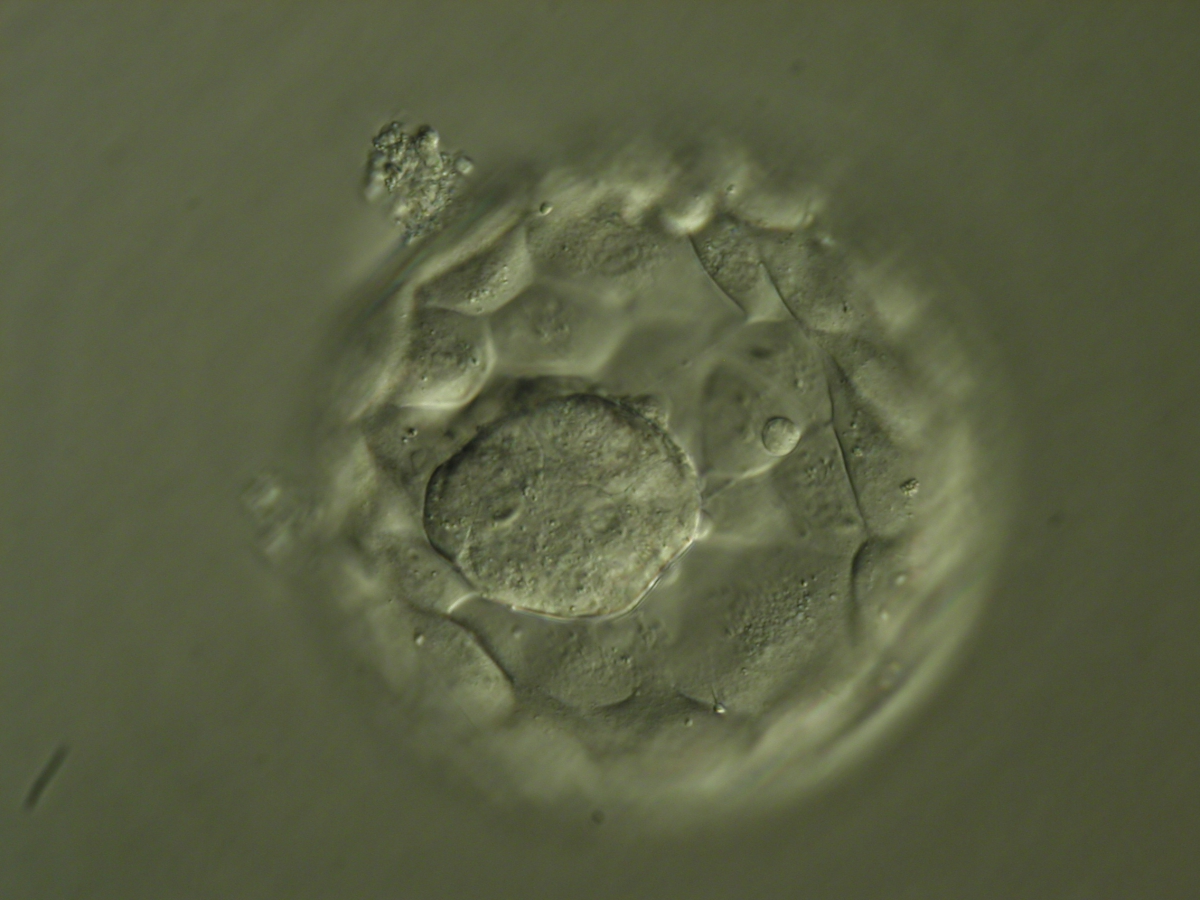
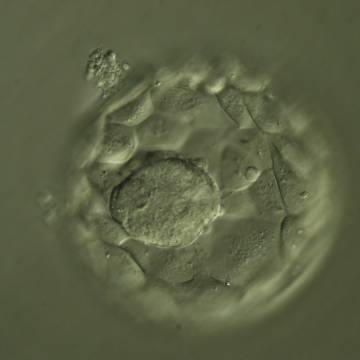
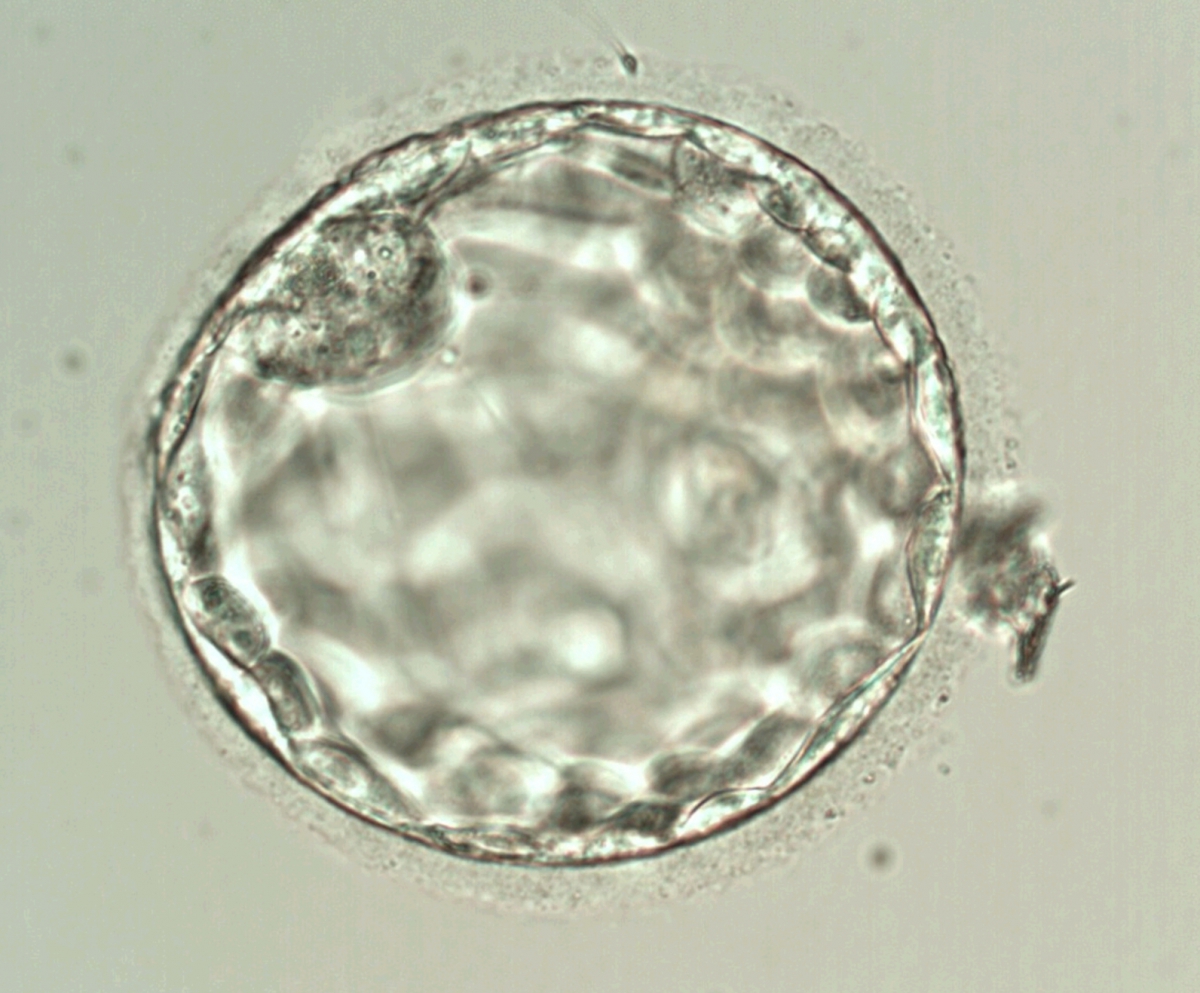
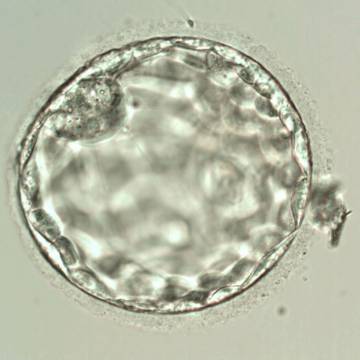
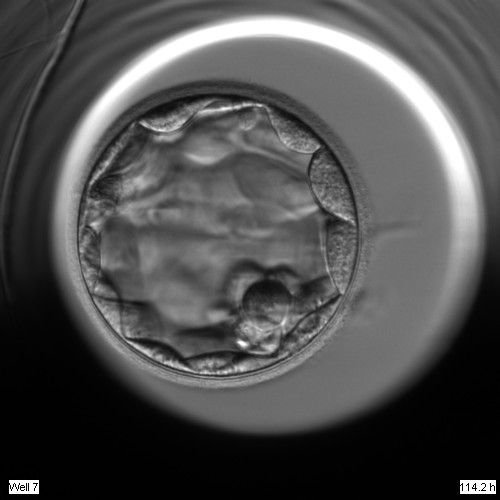
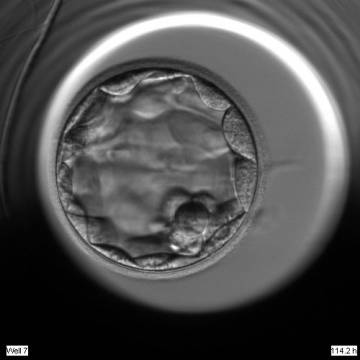
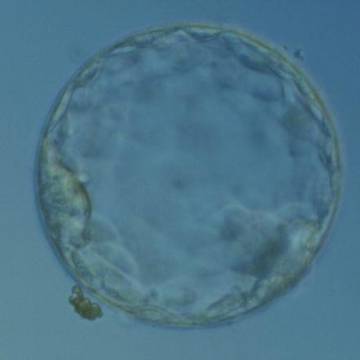
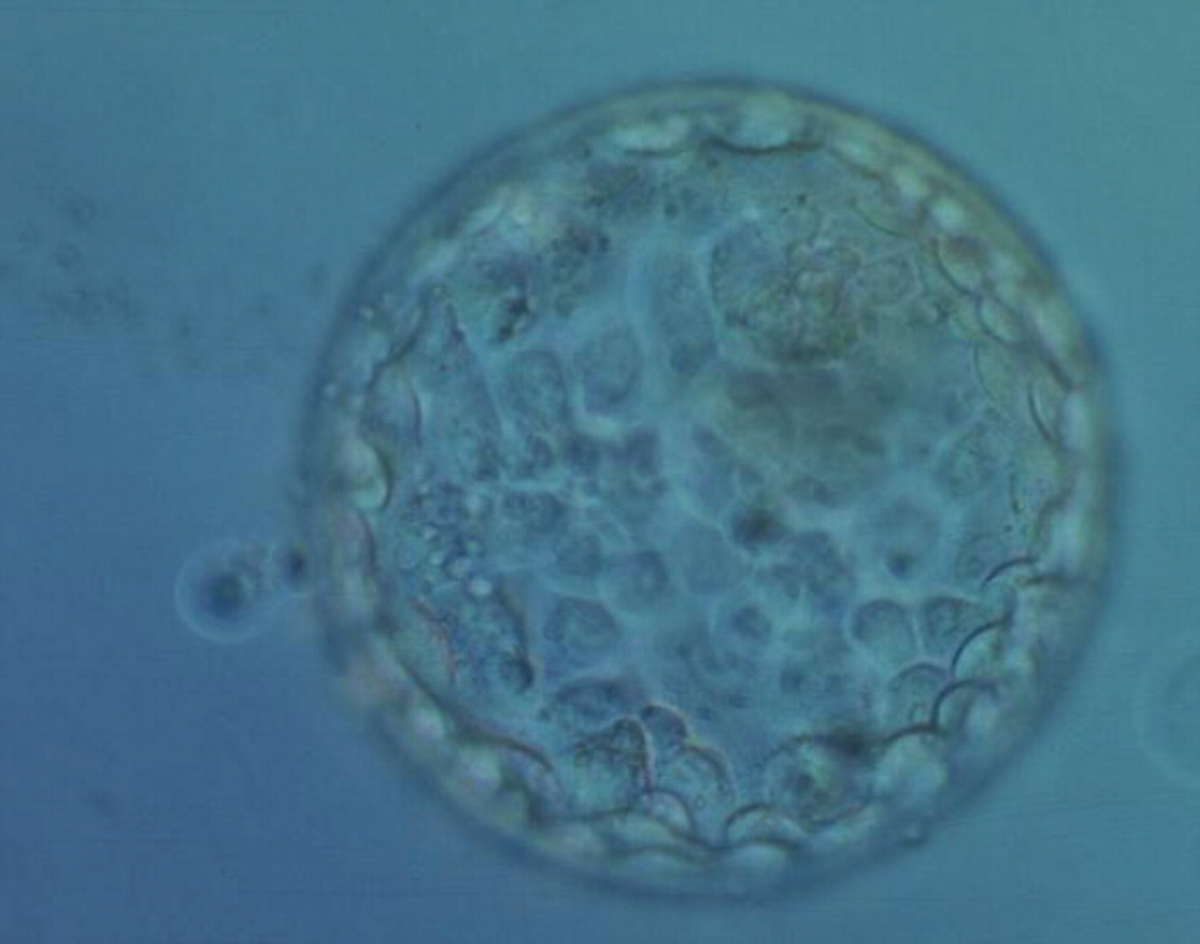
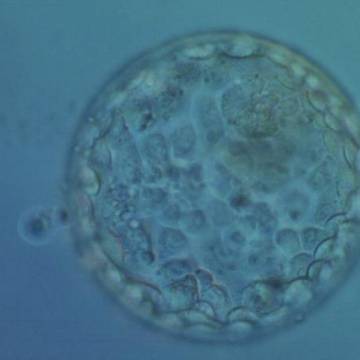
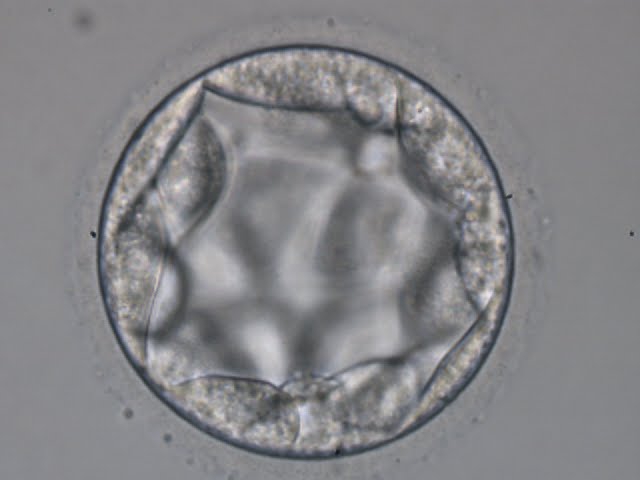
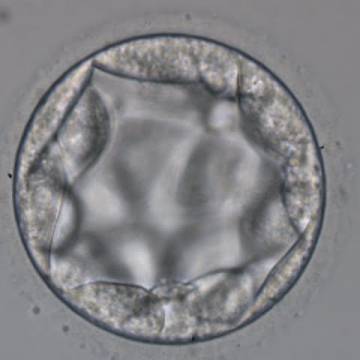
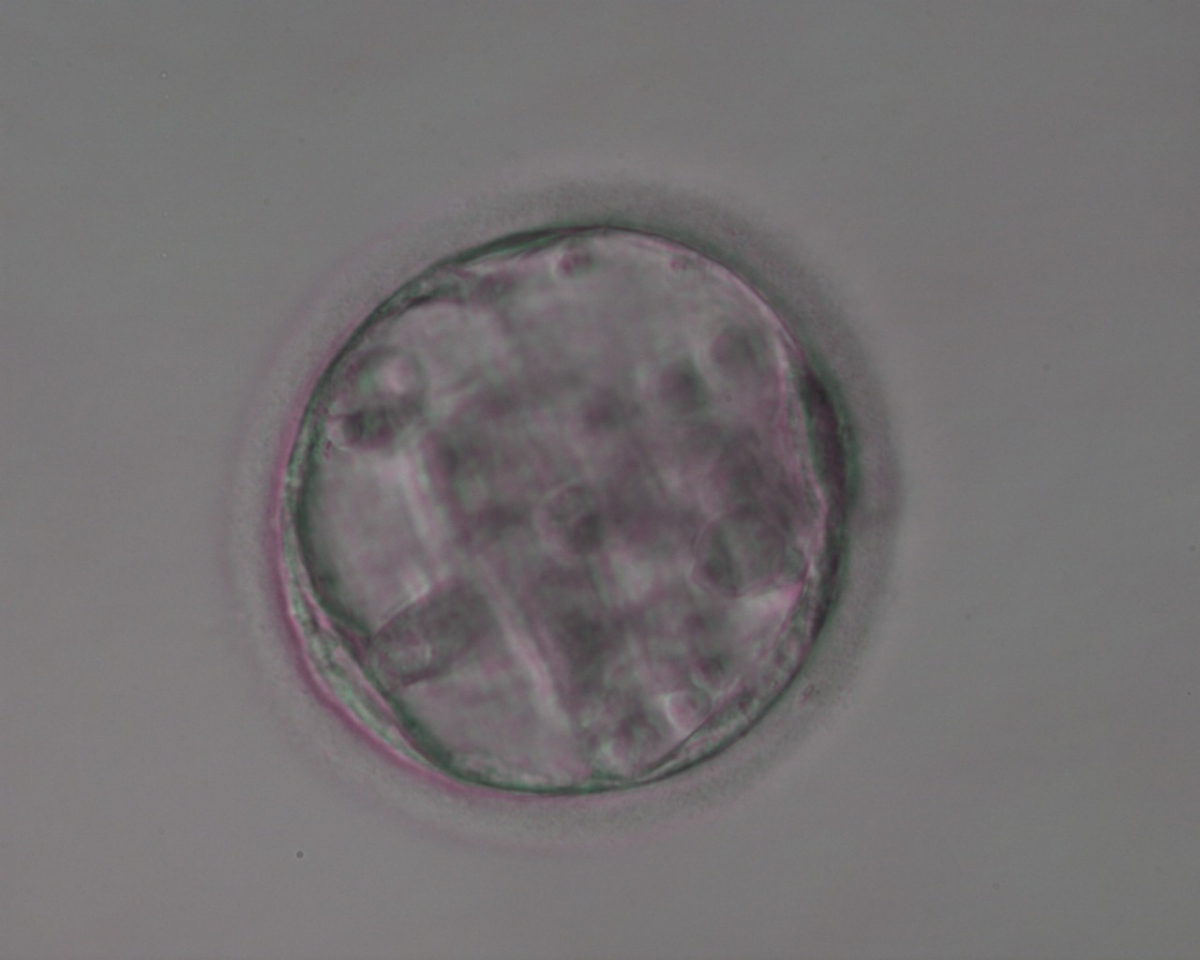
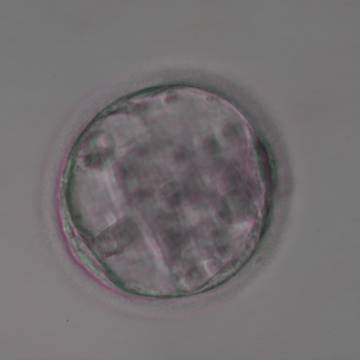
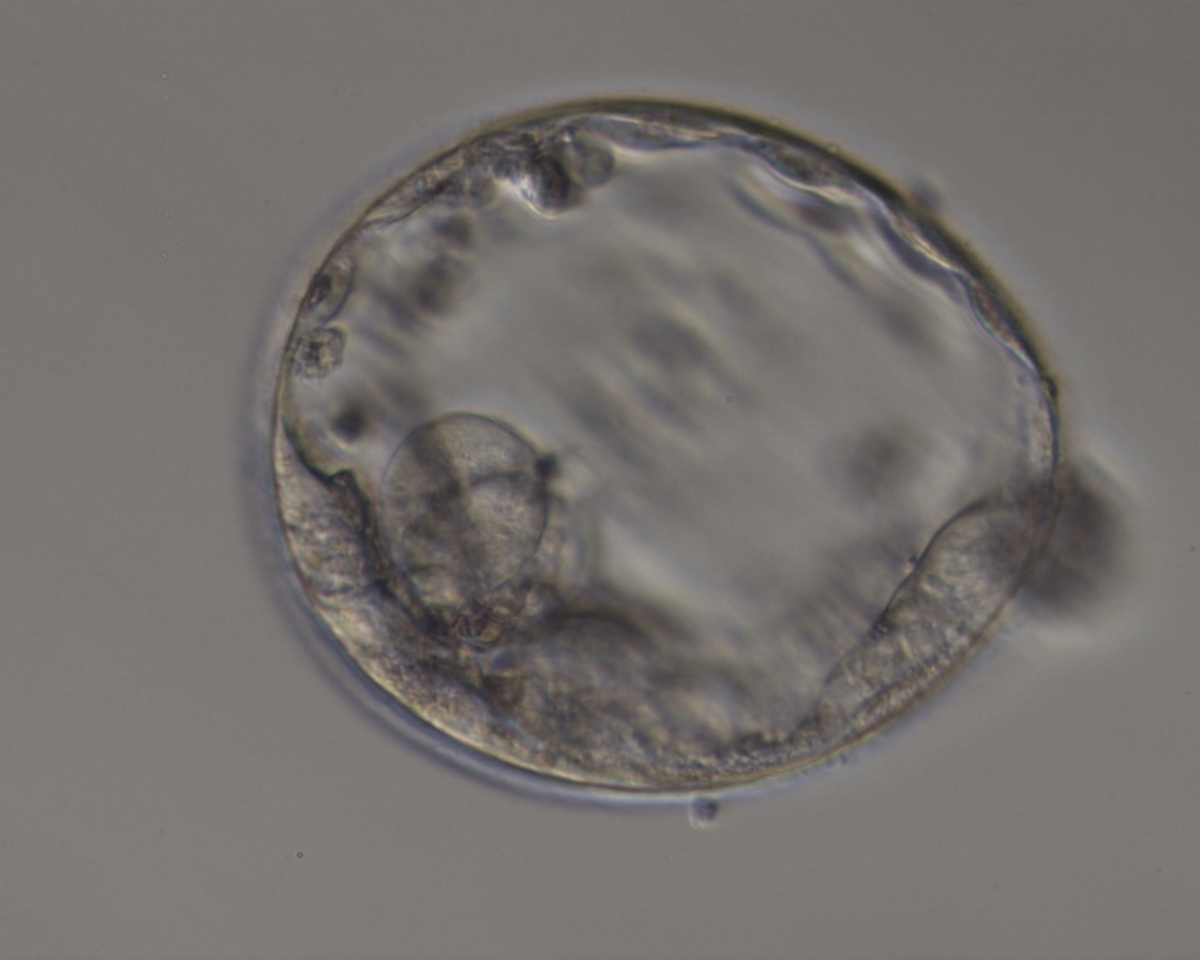
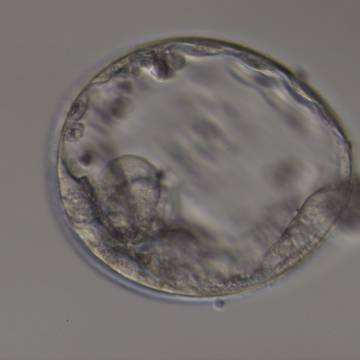
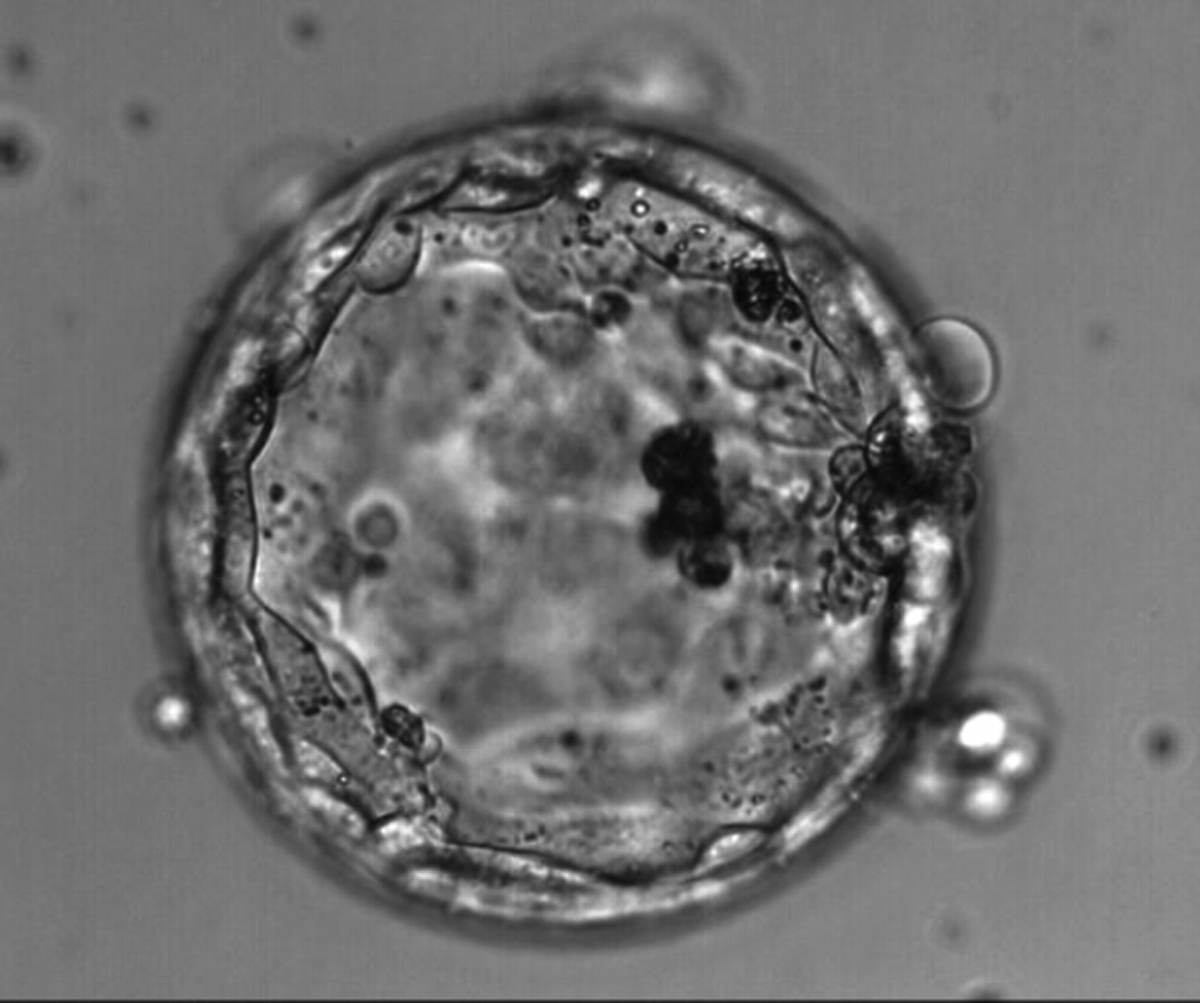
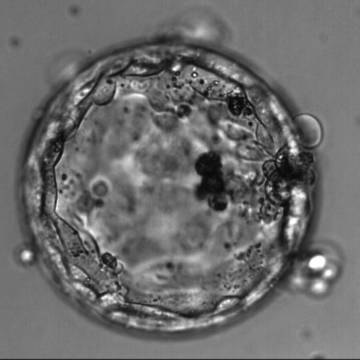
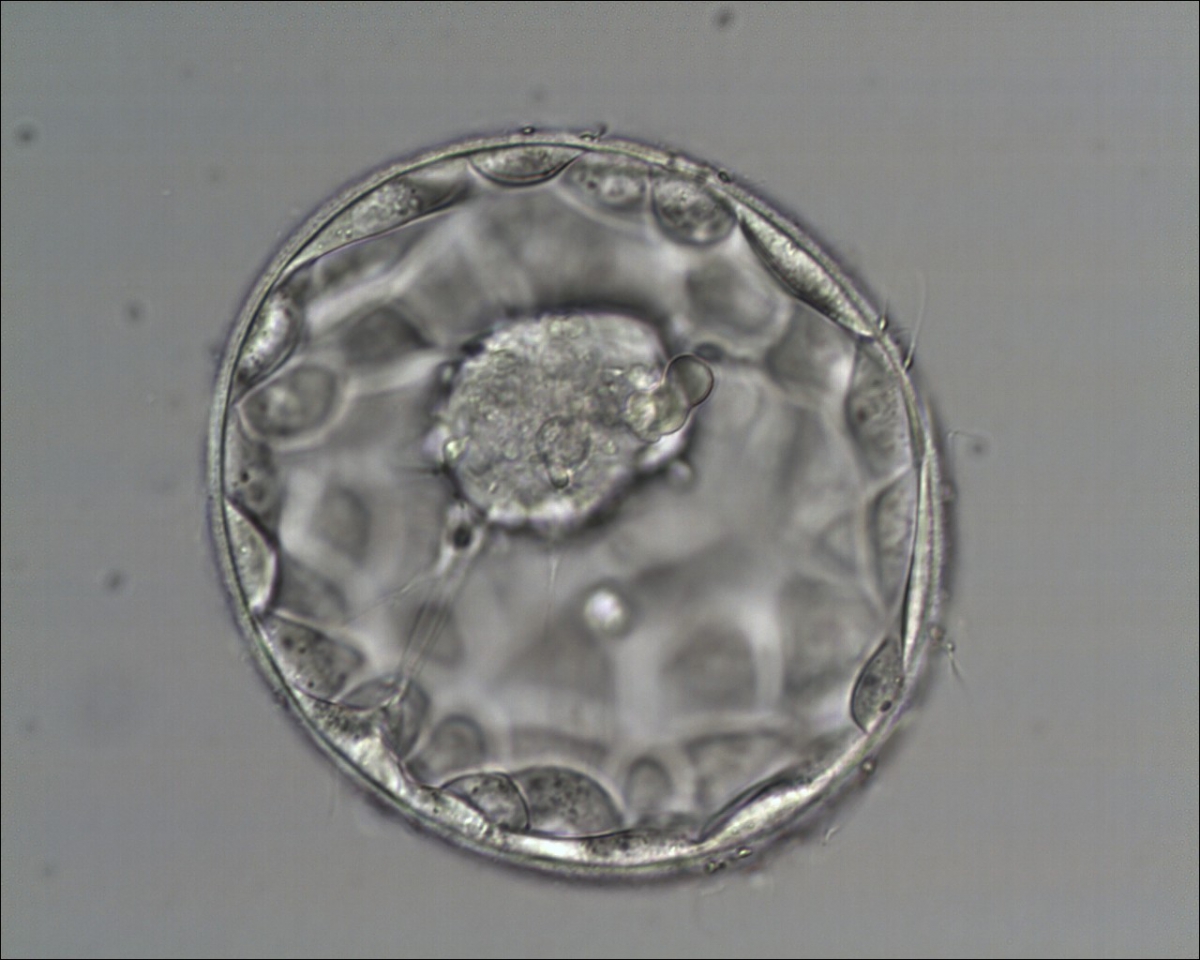
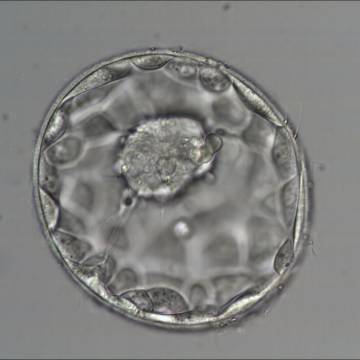
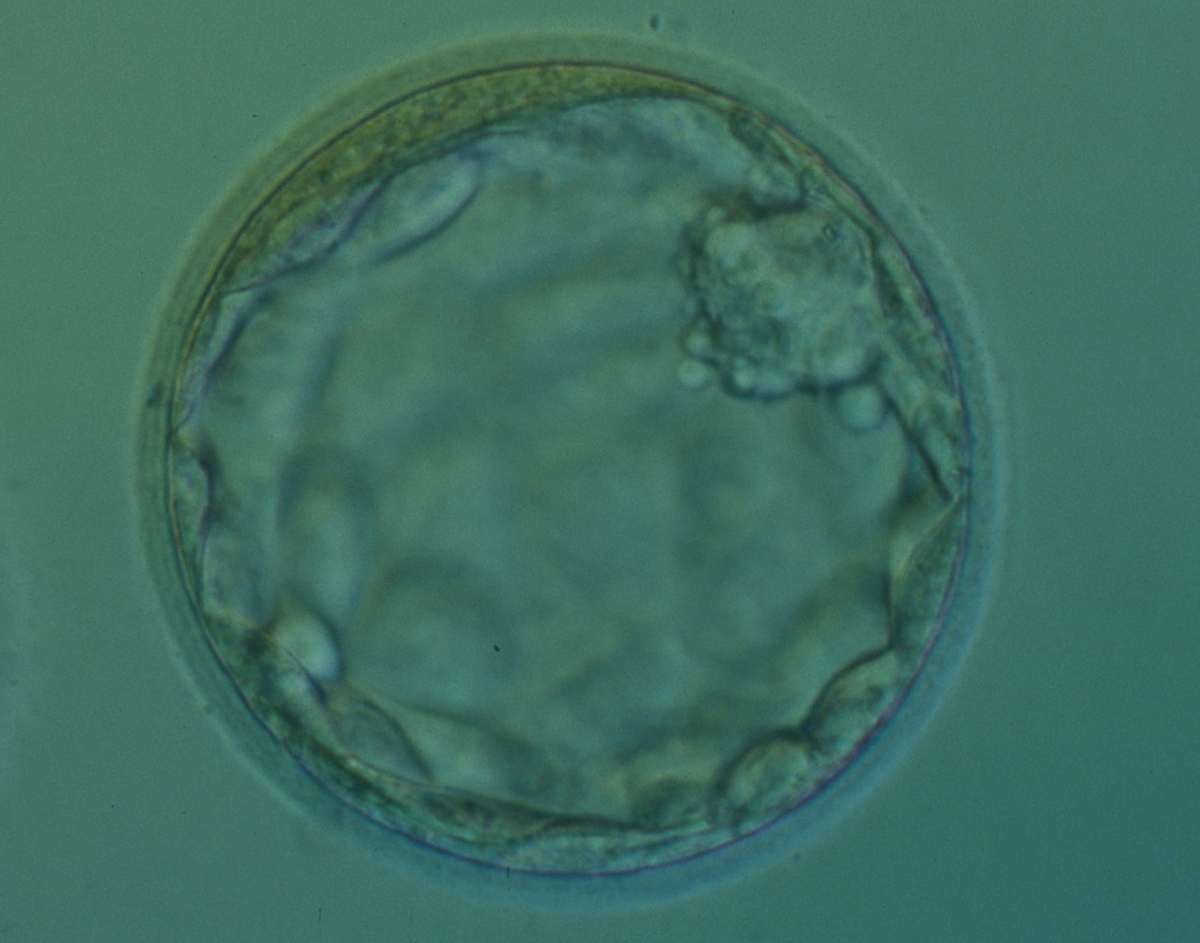
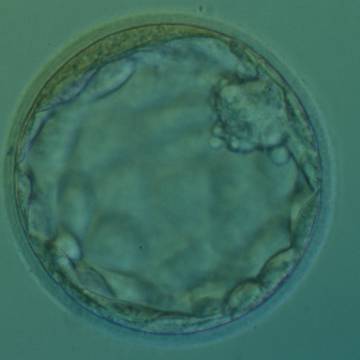
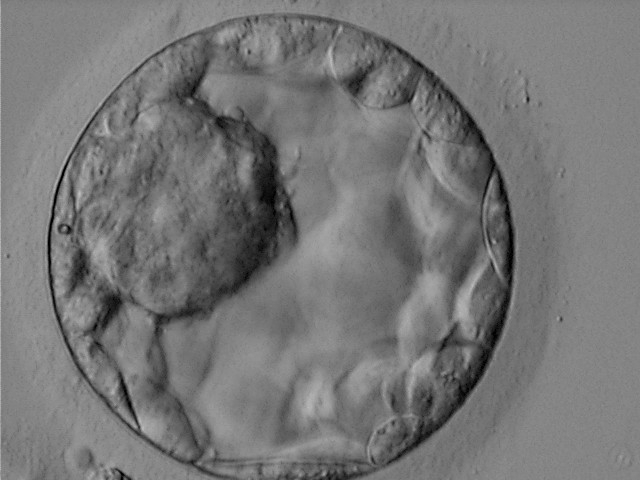
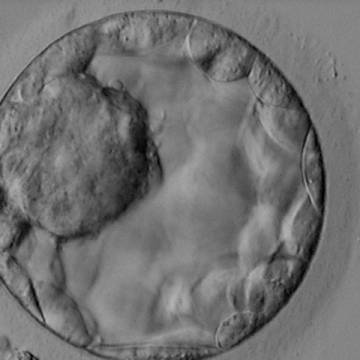
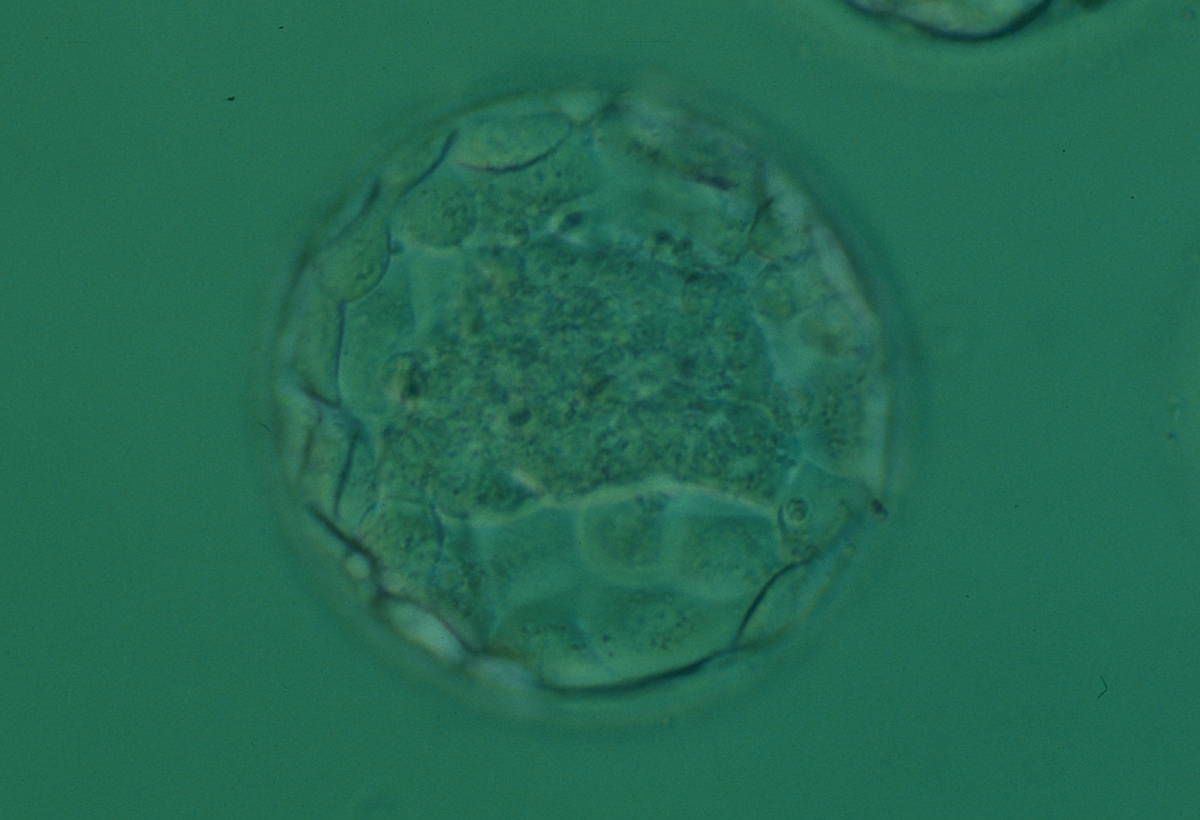
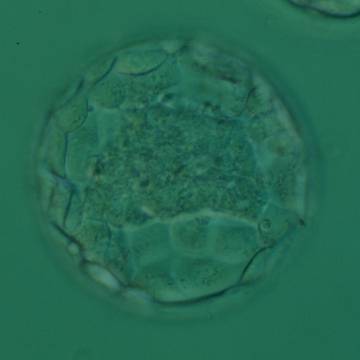
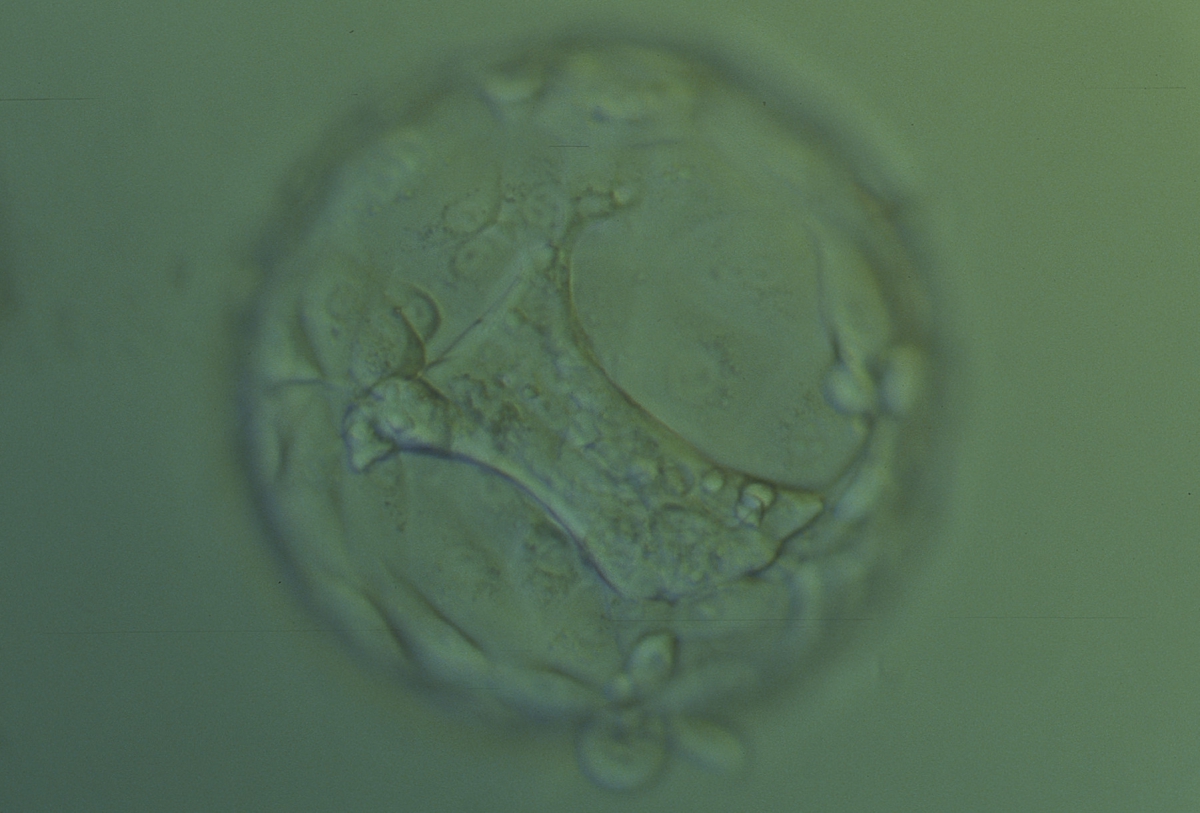
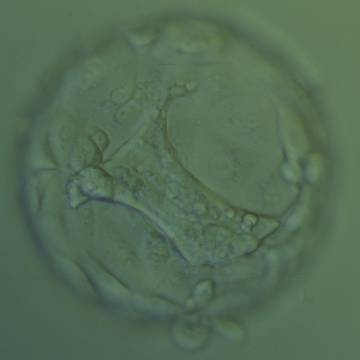
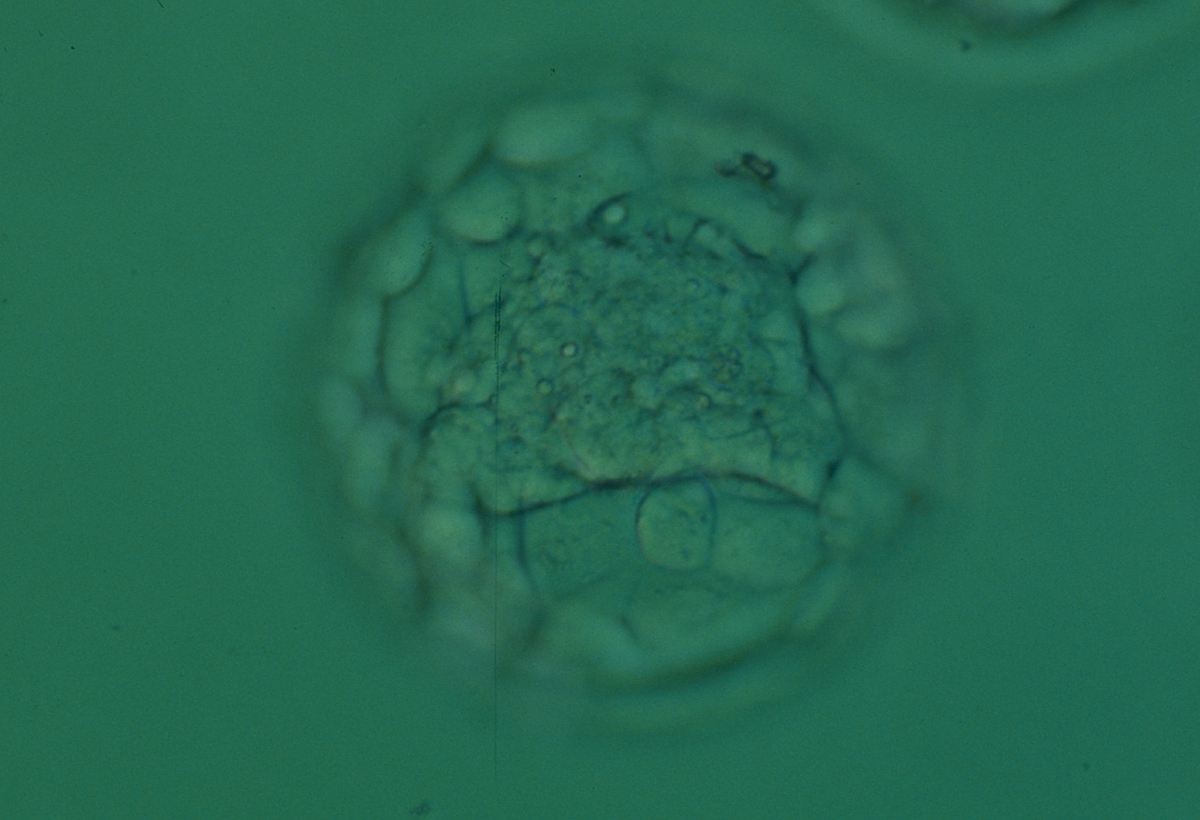
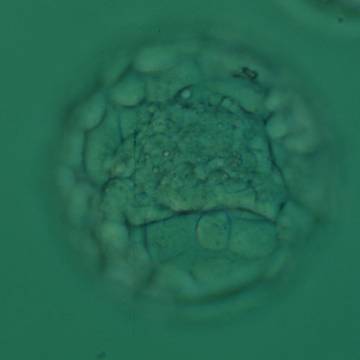
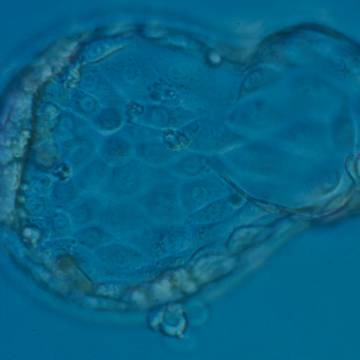
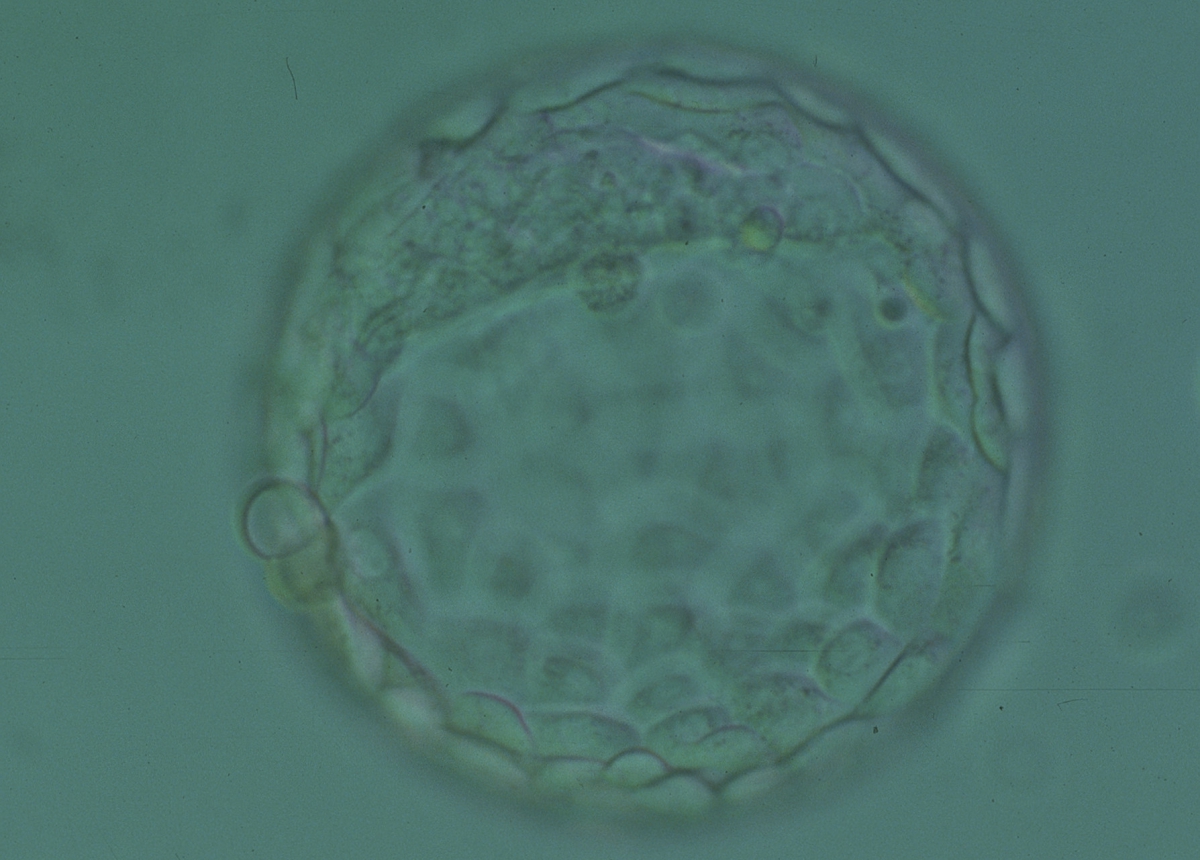
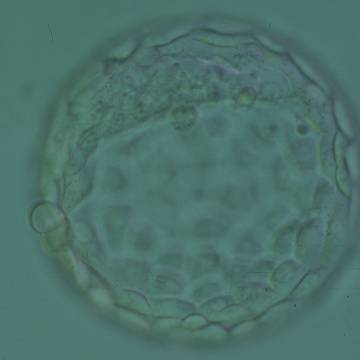
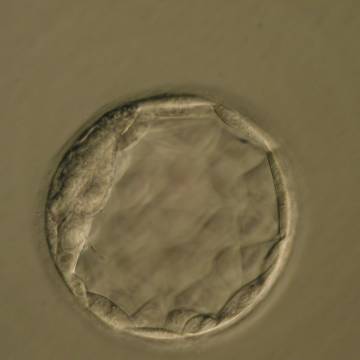
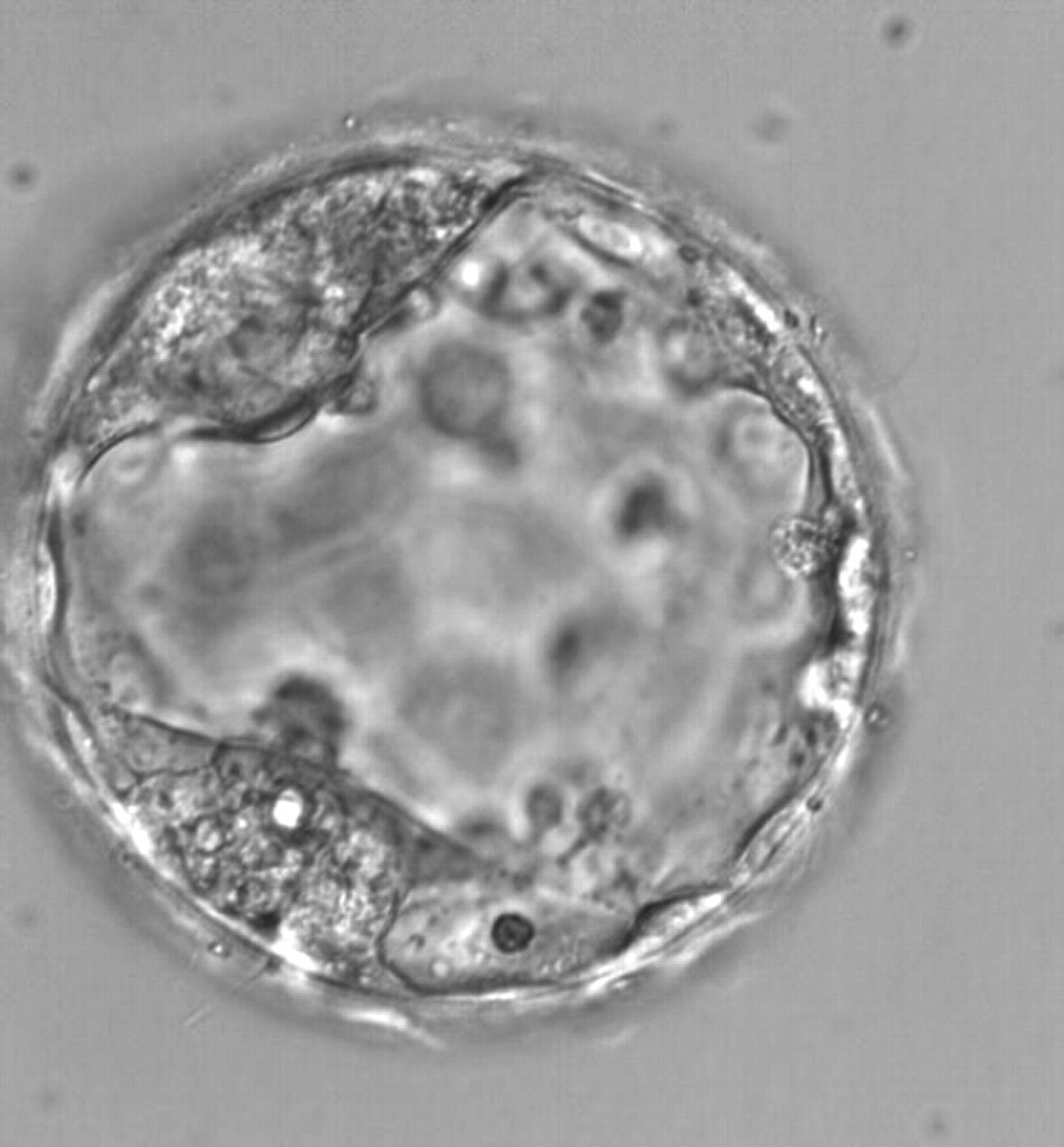
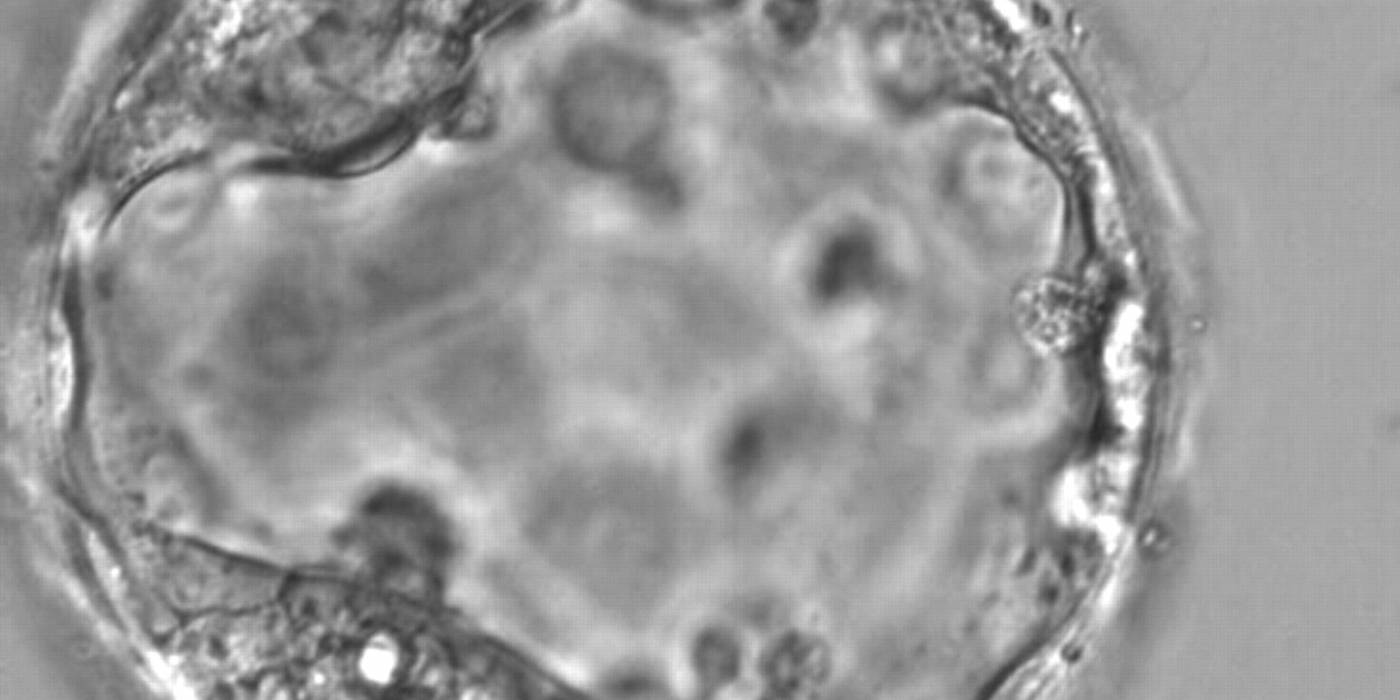
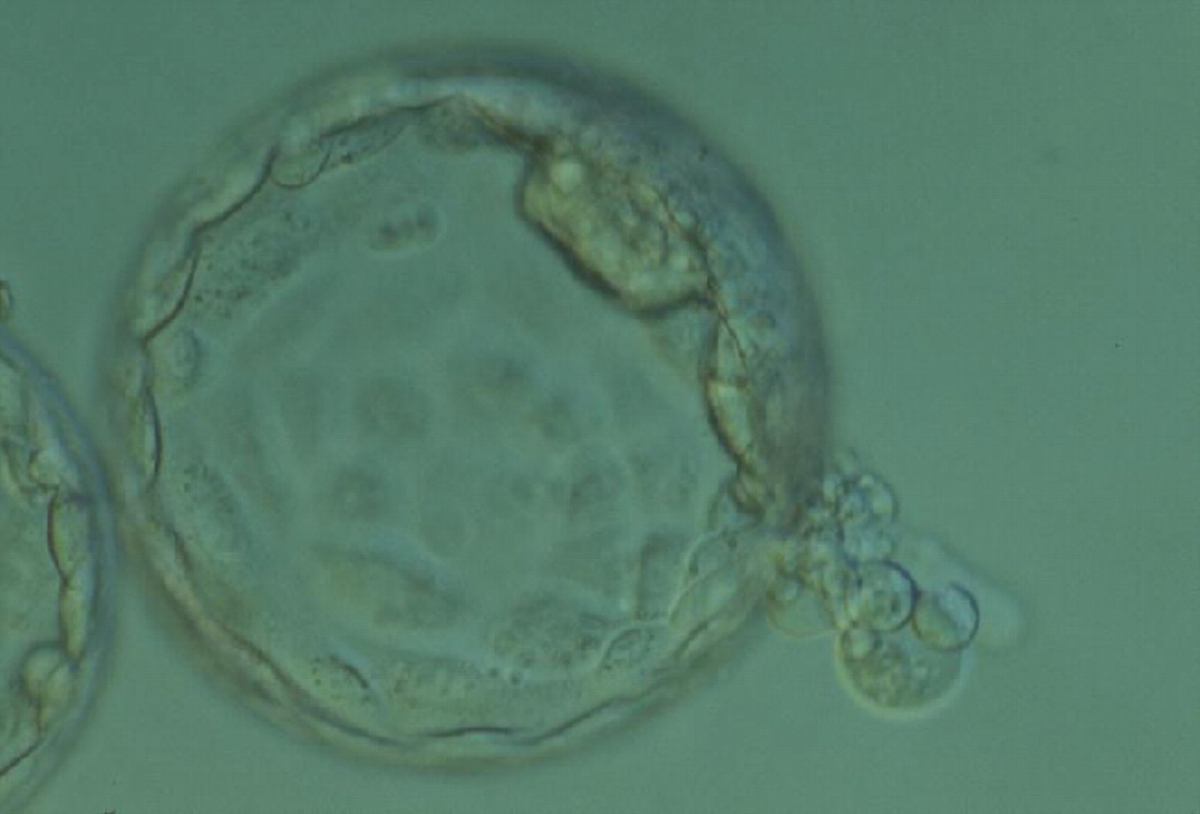
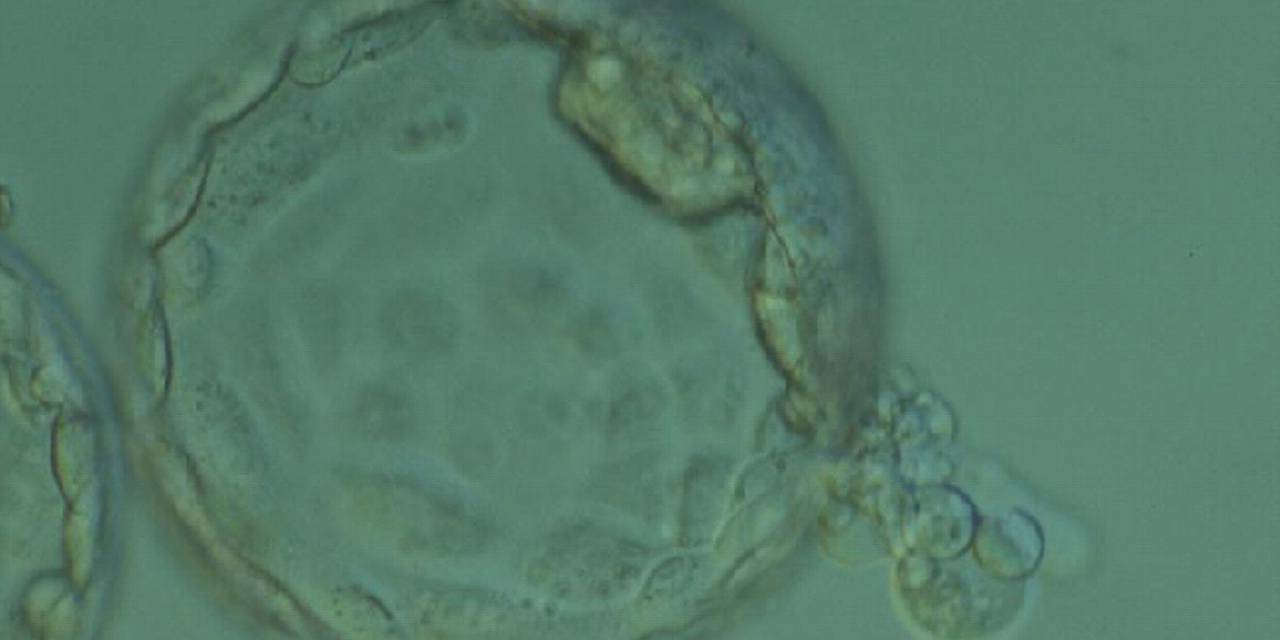
Once the blastocyst has reached an expansion grade of 3 or more, a clear distinction can be made between the two newly formed cell populations. The outer cells of the blastocyst, forming the blastocyst structure itself are called the TE cells and the cells located inside the blastocoel, often forming a cell clump at one pole of the blastocyst, are called the ICM cells. The destiny of the ICM is to become the embryo proper and its associated extra-embryonic structures. Morphologically, the ICM can range from being very large with tightly packed cells to almost non-existent with loosely bound cells. Accordingly, the ICM has been categorized into three morphological categories (Gardner and Schoolcraft, 1999; Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology, 2011). The best ICM category (1) contains many cells that are tightly packed together (Figs 342–345), the middle ICM category (2) is composed of several cells that are loosely grouped (Figs 346–349) and the worst category (3) describes an ICM that contains very few cells that are loosely bound (Figs 350–353). The number of cells composing the ICM can vary as well as the morphology of the cells within the ICM. Several studies have shown a positive correlation between the morphological appearance of the ICM to clinical outcome, the hypothesis being that the larger the ICM the better the chances of a successful implantation (Balaban et al., 2000; Richter et al., 2001).
The shape of the ICM has been observed to be quite variable in appearance. It has been reported that the optimal shape of the ICM with respect to implantation potential is more oval in shape rather than the rounder or more elongated forms (Richter et al., 2001). The shape of the ICM could in this instance reflect the number of cells involved. This Atlas identifies some further variability in shape and has classified the ICM into mushroom shaped (Figs 354–356), stellate shaped (Figs 357–359) and crescent shaped (Figs 360–362), the significance of the shape variations to implantation potential being unknown.
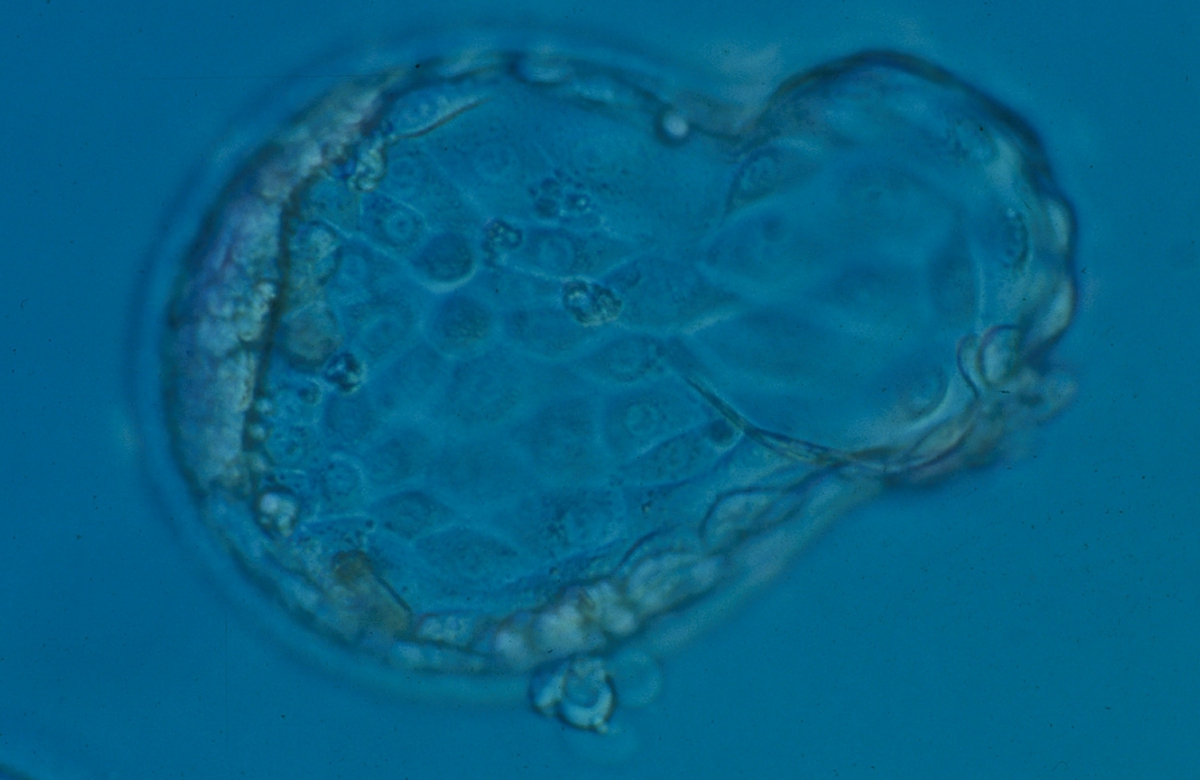
An occasional observation is the presence of two separate ICMs within the blastocyst. Chida (2000) reported the incidence of a double ICM as 3.1% for in vitro cultured mouse blastocysts and the majority of these developed after hatching into trophoblastic outgrowth formation with a double ICM. They found that the frequency of a double ICM was significantly higher in in vitro fertilized blastocysts than that for in vivo fertilized blastocysts (0.6%). Therefore, it was hypothesized that the in vitro fertilization conditions could influence the incidence of double ICMs and as a consequence the incidence of monozygotic twinning.
Meintjes et al. (2001) reported a human IVF monozygotic pregnancy resulting from a blastocyst with two distinct ICMs. In fact, it was a triplet pregnancy obtained after transfer of two Day 5 blastocysts. The monozygotic pregnancy was found to be dichorionic diamniotic, indicating the splitting of the TE as well. Payne et al. (2007) presented at the ESHRE congress in 2007 a time-lapse recording of frozen–thawed human embryos cultured to the blastocyst stage. Out of 26 blastocysts, two had a double ICM already evident at the early blastocyst stage. Remarkably, the second ICM seemed to be developed after ectopic adhesion of ICM cells to the opposing TE cells following early collapse of the blastocoel cavity. This Atlas illustrates two incidences where the ICM appears to be duplicated (Figs 363 and 364).
Article references:
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 2011;26:1270-1283.
Abstract/FREE Full Text
Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Blastocyst quality affects the success of blastocyst-stage embryo transfer. Fertil Steril 2000;74:282-287.
CrossRef | Medline | Web of Science | Google Scholar
Chida S. Monozygous double inner cell masses in mouse blastocysts following fertilization in vitro and in vivo. J In Vitro Fert Embryo Transf 2000;7:177-179.
Google Scholar
Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Toward Reproductive Certainty: Fertility and Genetics Beyond 1999. UK: Parthenon Publishing London; 1999. p. 378-388.
Google Scholar
Meintjes M, Guerami AR, Rodriguez JA, Crider-Pirkle SS, Madden JD. Prospective identification of an in-vitro-assisted monozygotic pregnancy based on a double-inner-cell-mass blastocyst. Fertil Steril 2001;76:S172-S173.
Google Scholar
Payne D, Okuda A, Wakatsuki Y, Takeshita C, Iwata K, Shimura T, Yumoto K, Ueno Y, Flaherty S, Mio Y. Time-lapse recording identifies human blastocysts at risk of producing monozygotic twins. Hum Reprod 2007;22(Suppl.):i9-i10.
FREE Full Text
Richter KS, Harris DC, Daneshmand ST, Shapiro BS. Quantitative grading of a human blastocyst: optimal inner cell mass size and shape. Fertil Steril 2001;76:1157-1167.
CrossRef | Medline | Web of Science | Google Scholar