D. Cellular degeneration in blastocysts
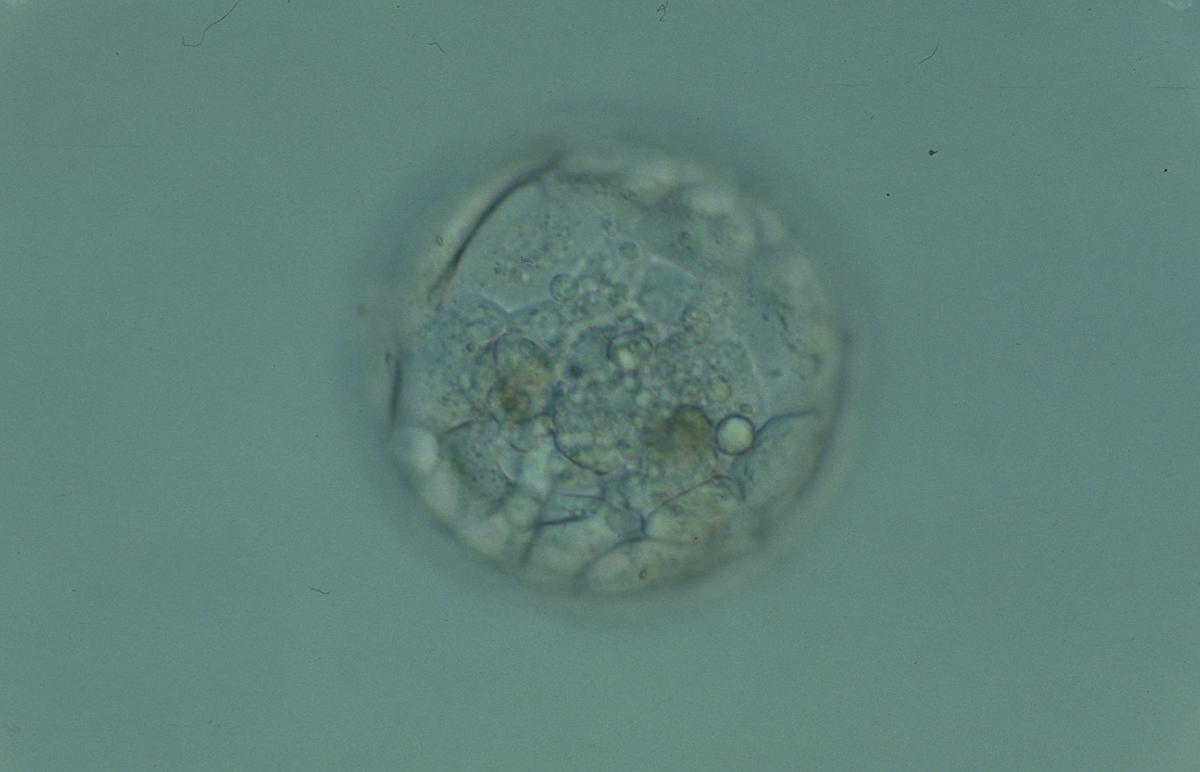
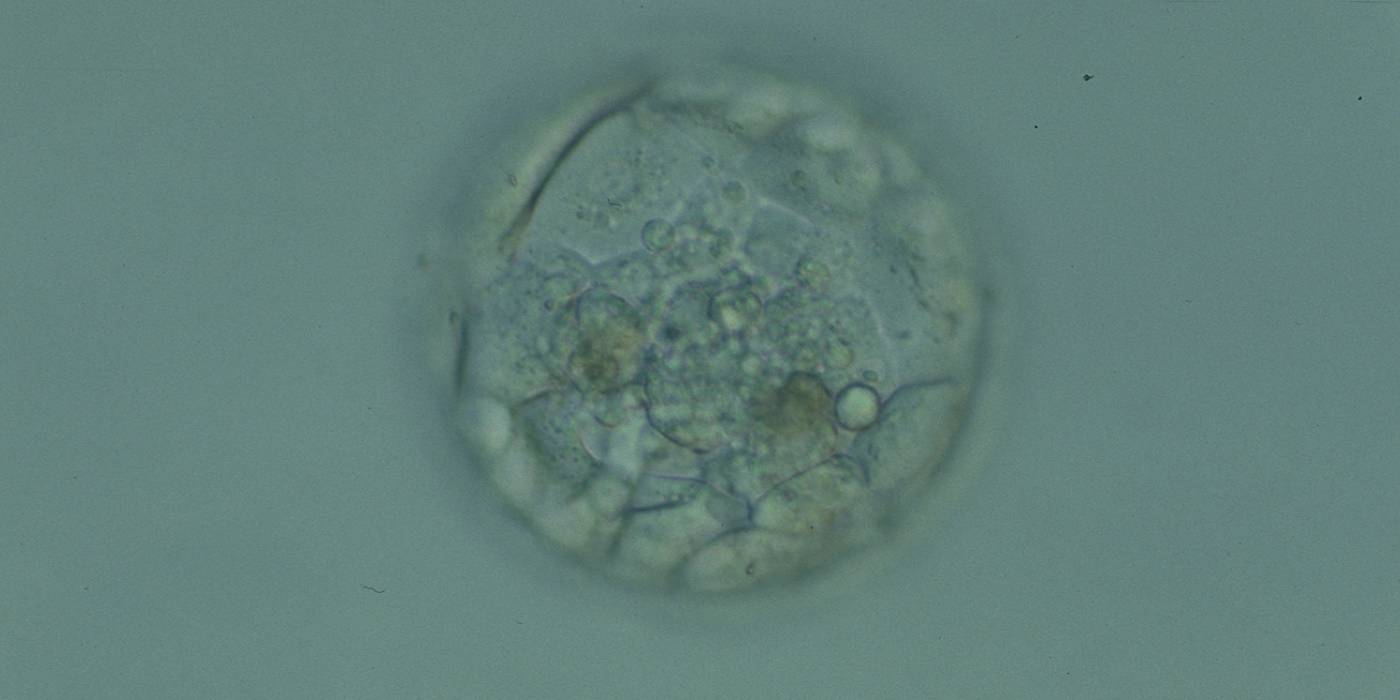
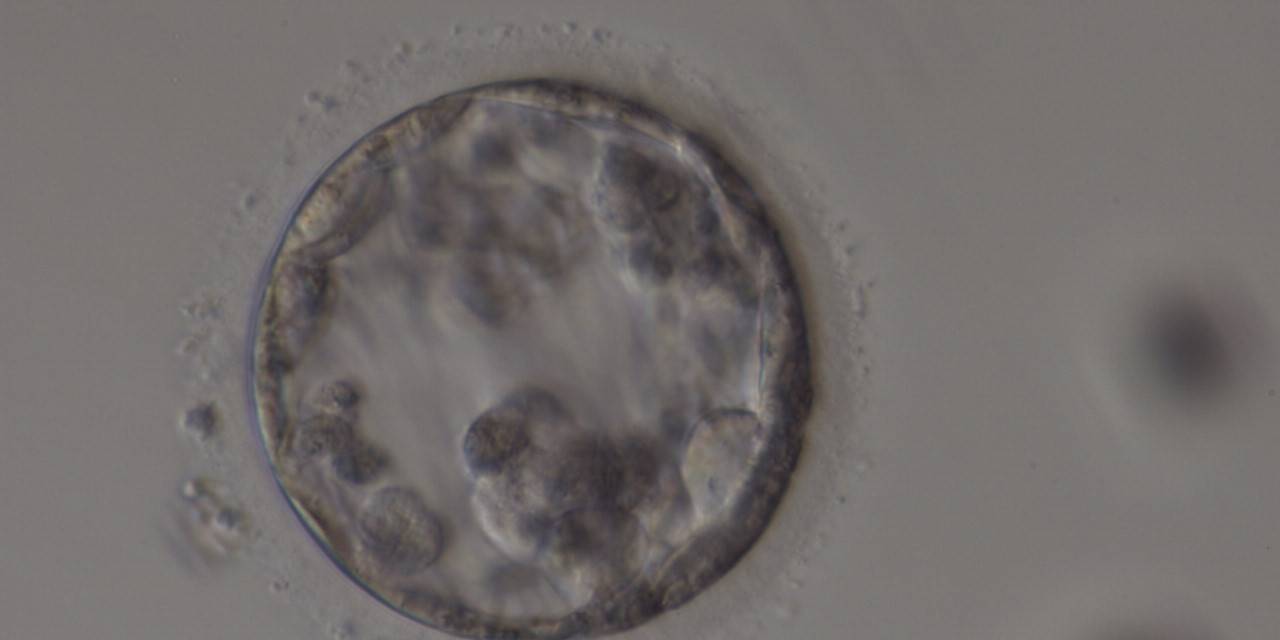
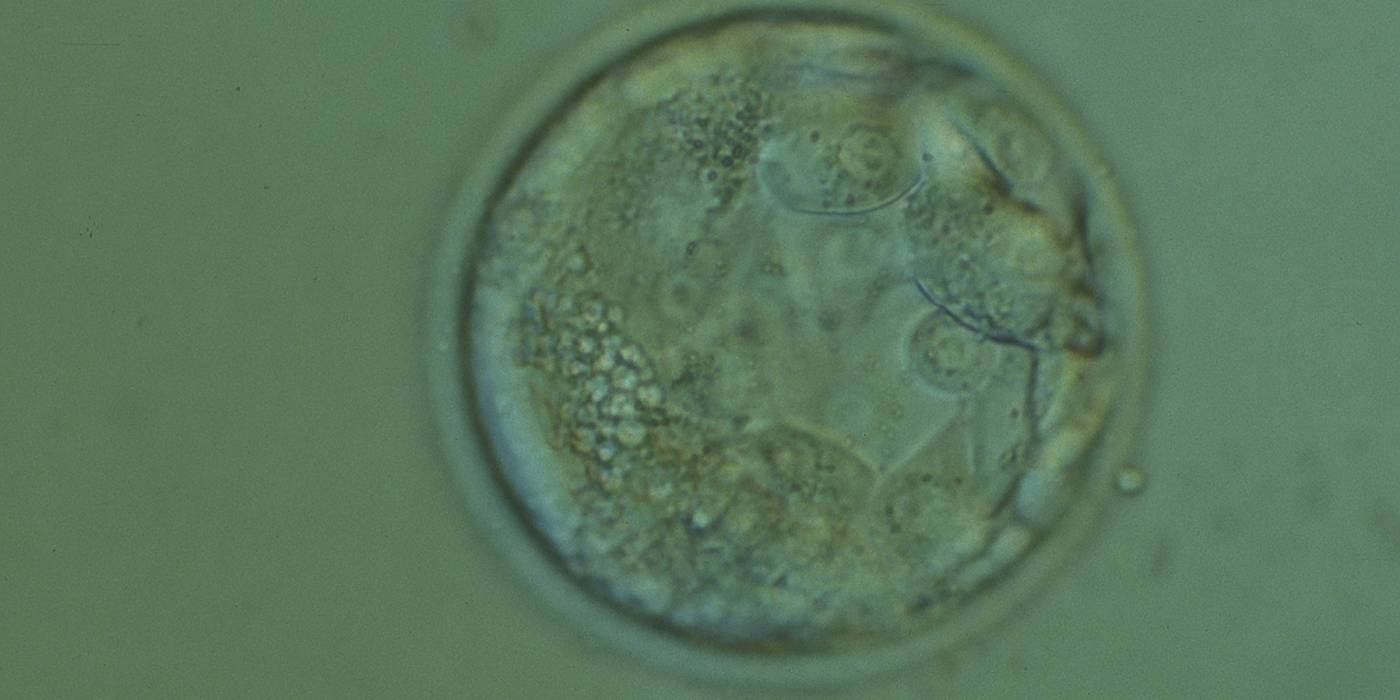
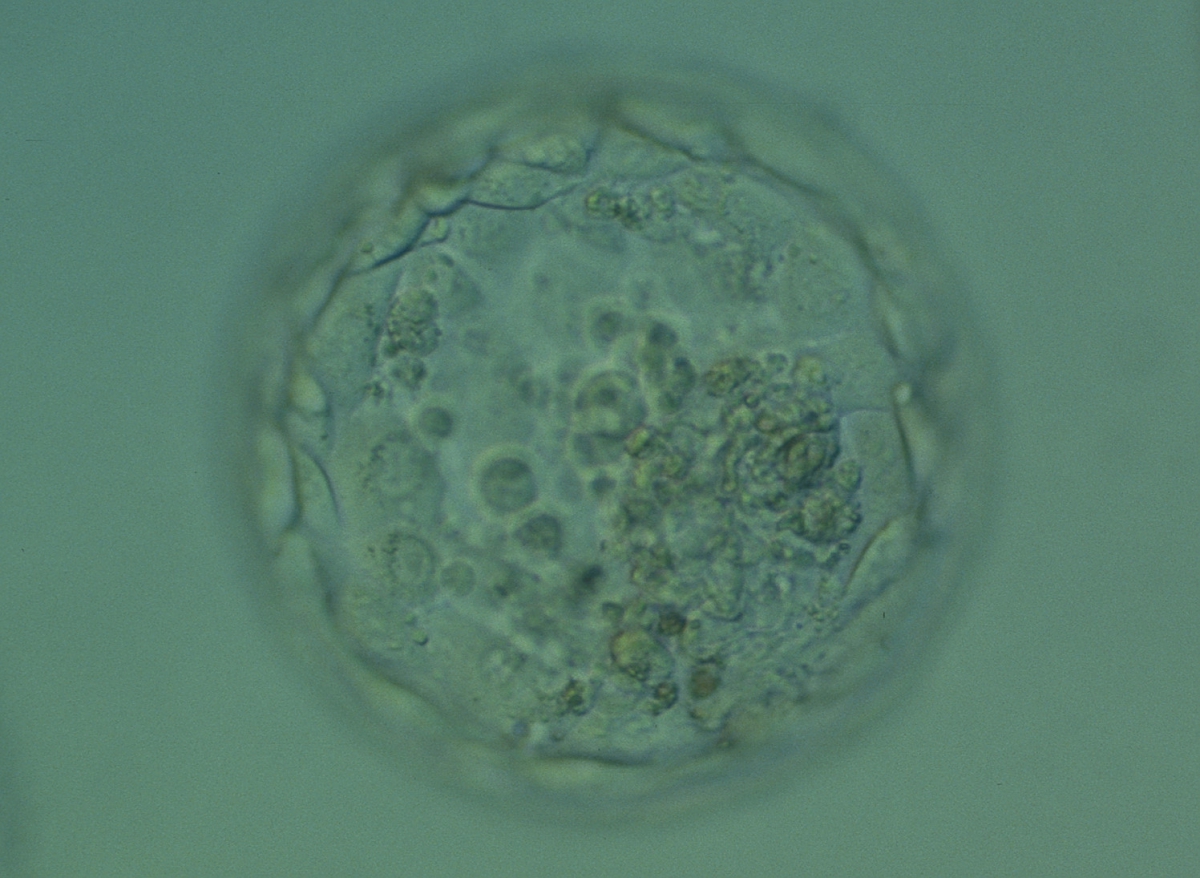
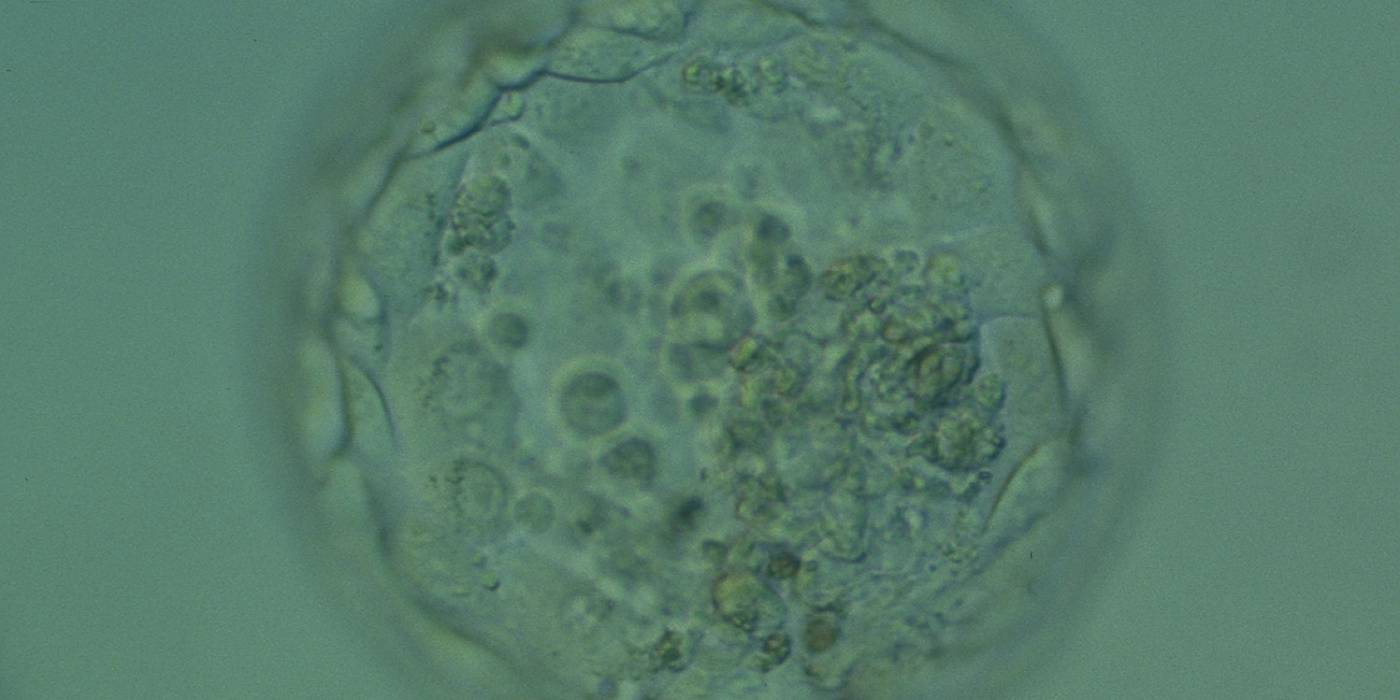
Cell death can occur by necrosis or apoptosis, two processes with different morphological features and significance. Necrosis involves swelling of cells and membrane rupture that follows irreversible damage (Wyllie, 1980). Cell death generally occurs by apoptosis, characterized by cellular shrinkage and involves aggregation of nuclear chromatin, condensation of the cytoplasm and indention of nuclear and cytoplasmic membranes (Figs 380–382). The nucleus becomes fragmented, the cell forms blebs and fragments into apoptotic bodies (Hardy 1997; 1999).
Hardy et al. (1989) showed by differential labelling of TE and ICM nuclei in supernumerary human blastocysts that the percentage of cell death was similar in both ICM and TE cells and increased with culturing blastocysts up to Day 7. The dead cell index was <10% for Days 5 and 6 good quality blastocysts but was increased up to 27.0 and 38.5% for Day 6 morphologically abnormal and polyspermic blastocysts, respectively. Dead cells within the blastocyst can be dissolved by the process of phagocytosis.
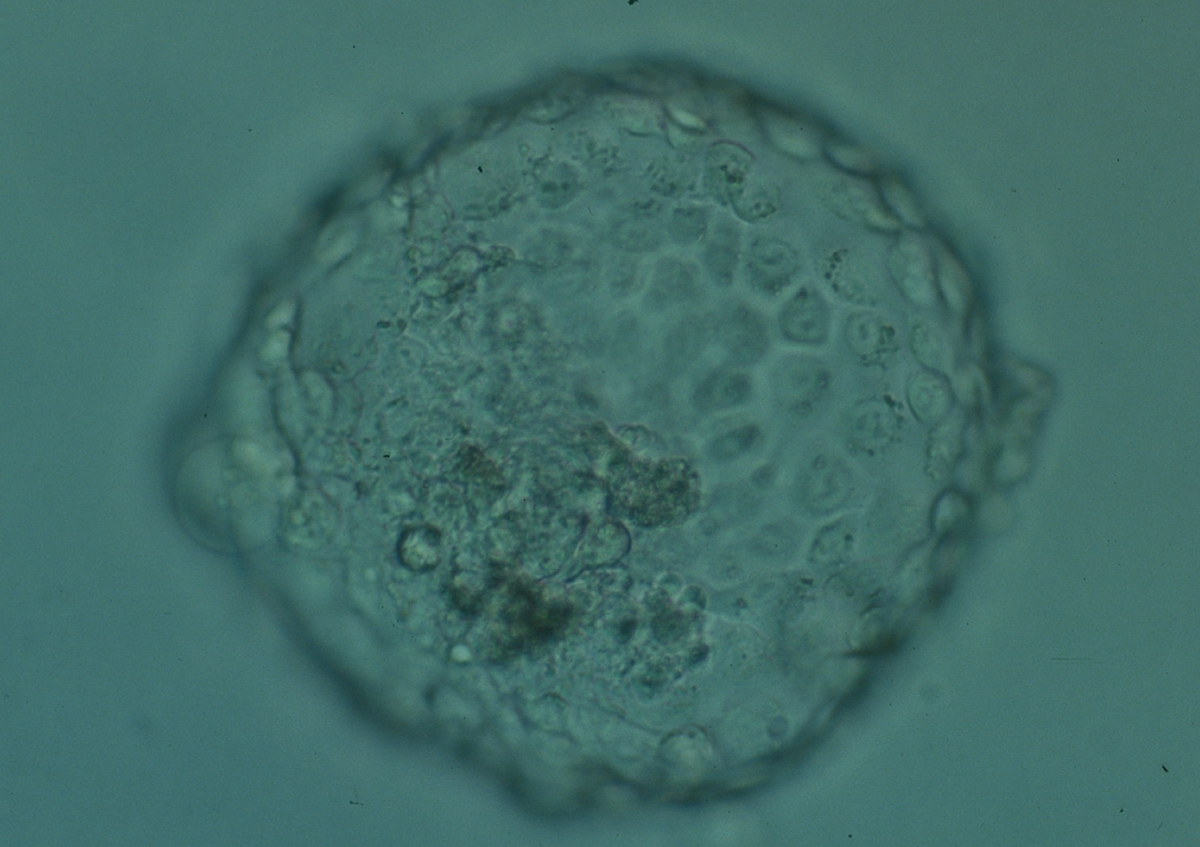
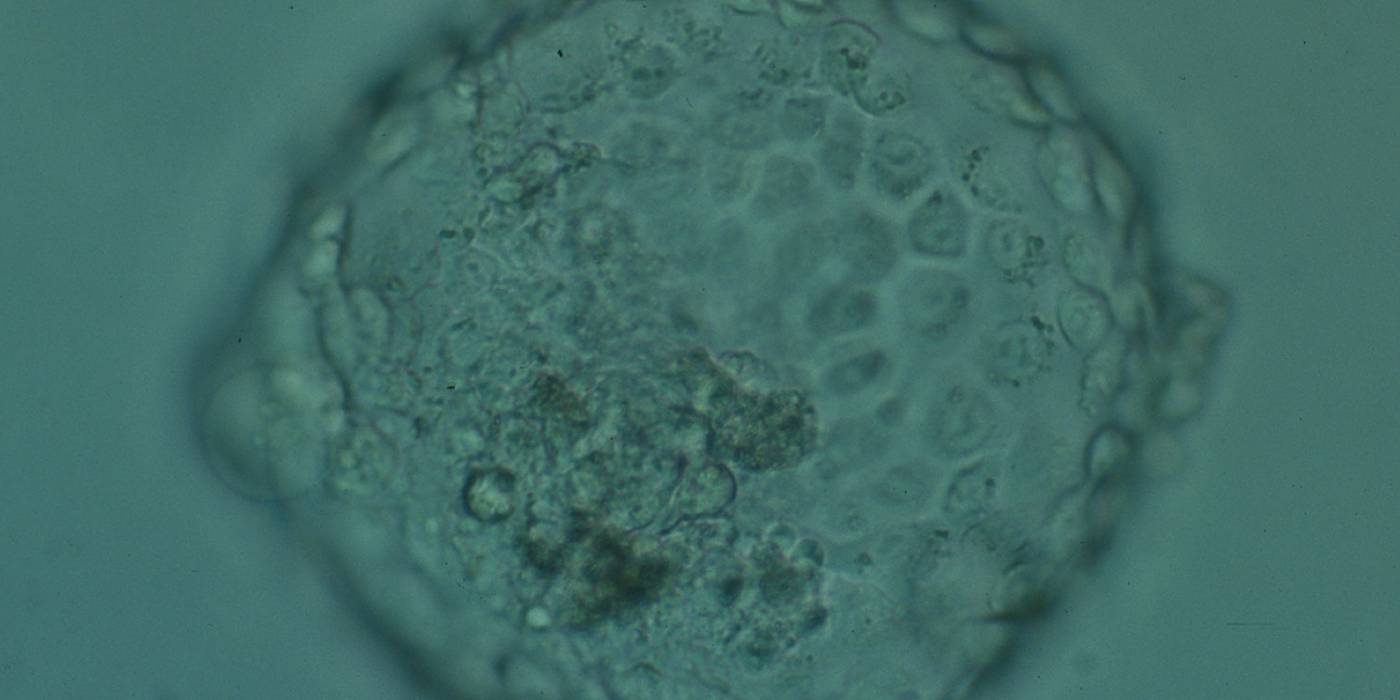
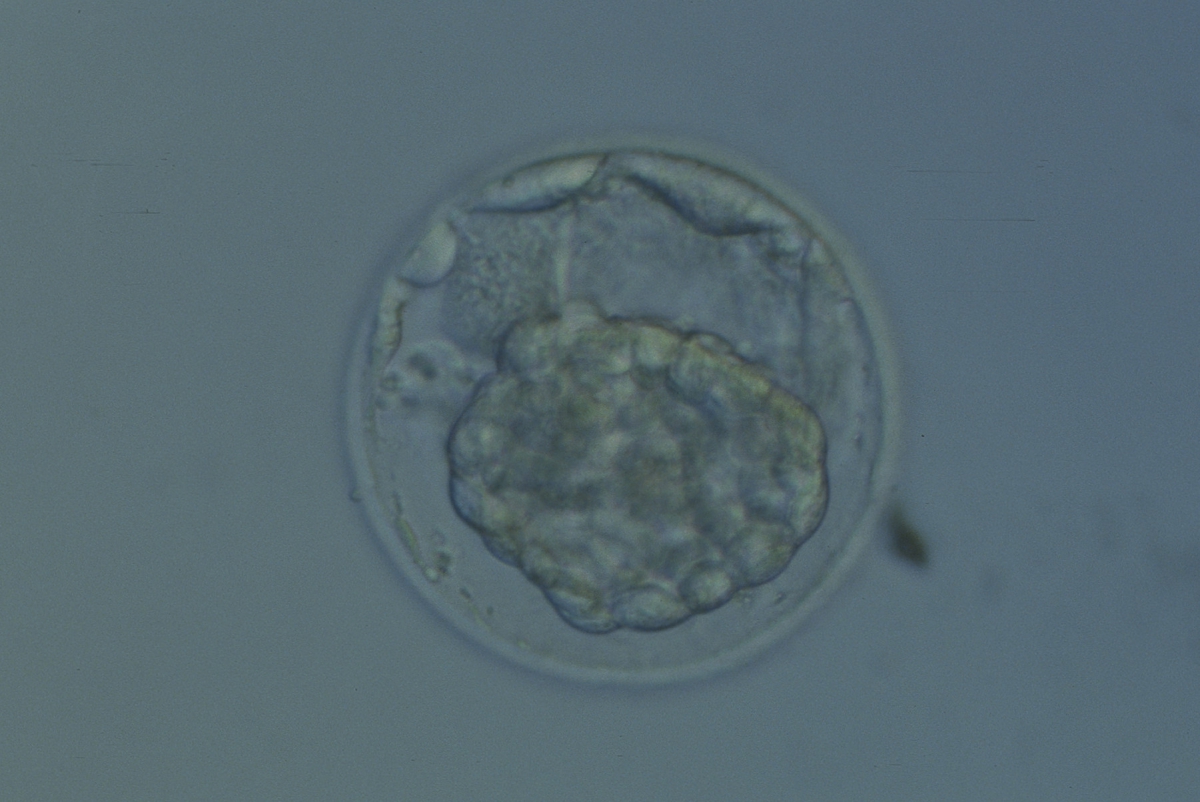
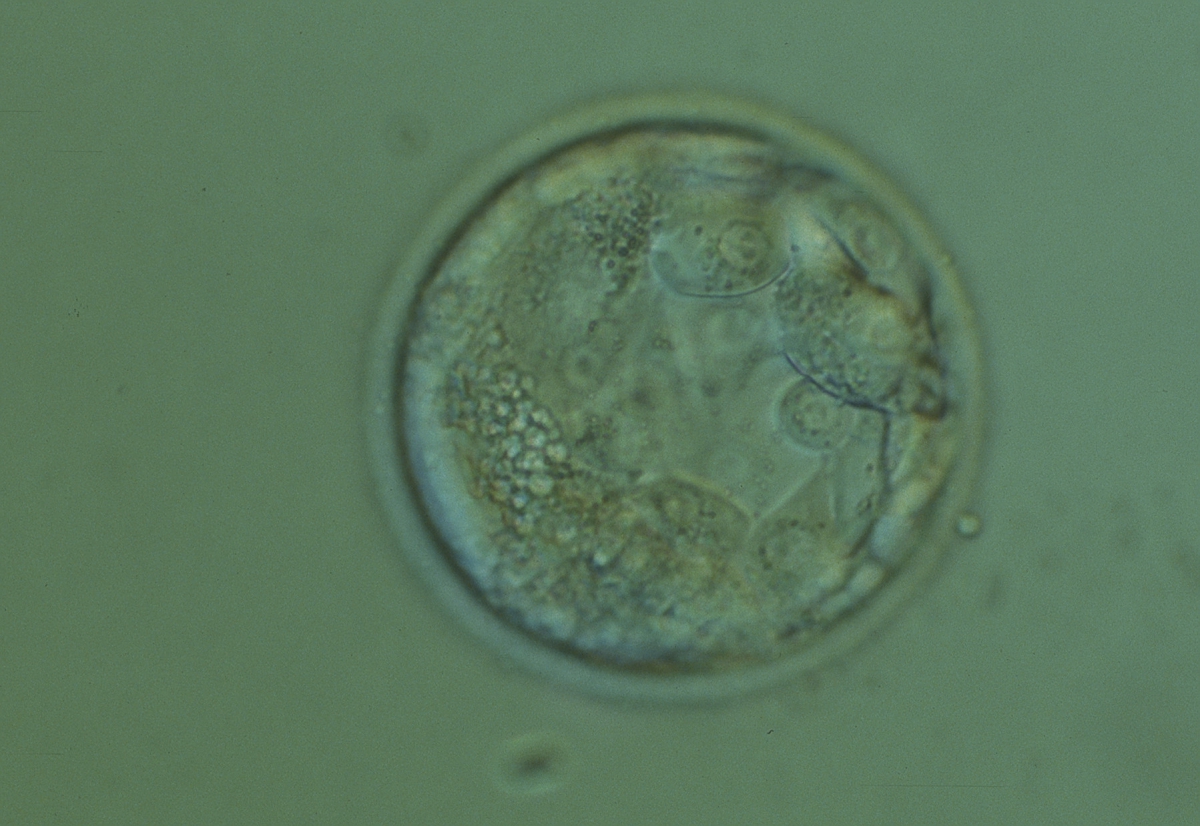
Isolated foci of degeneration should be distinguished from total degenerative change within the blastocyst as described and classified as BG3 blastocysts by Dokras et al. (1993; Fig. 382). These blastocysts are irredeemable, continue to degenerate further and have no potential to implant and develop to term.
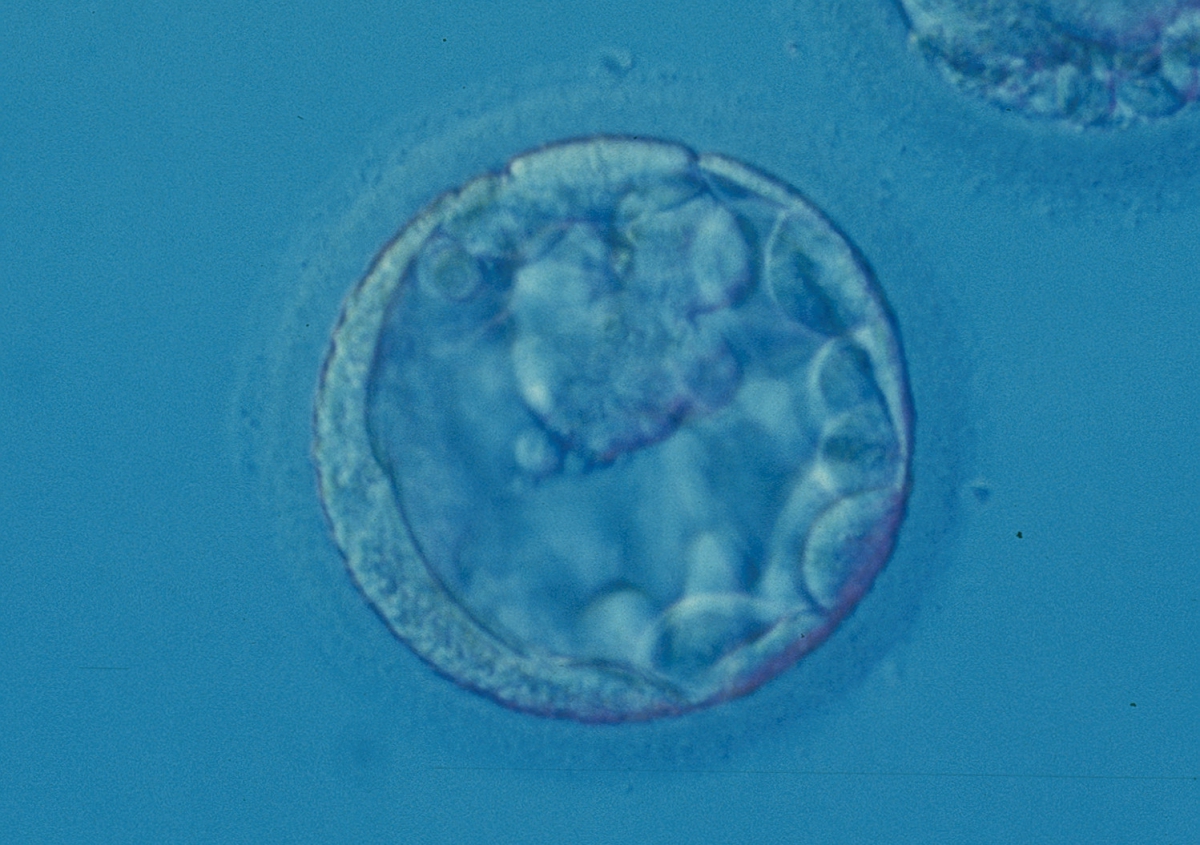
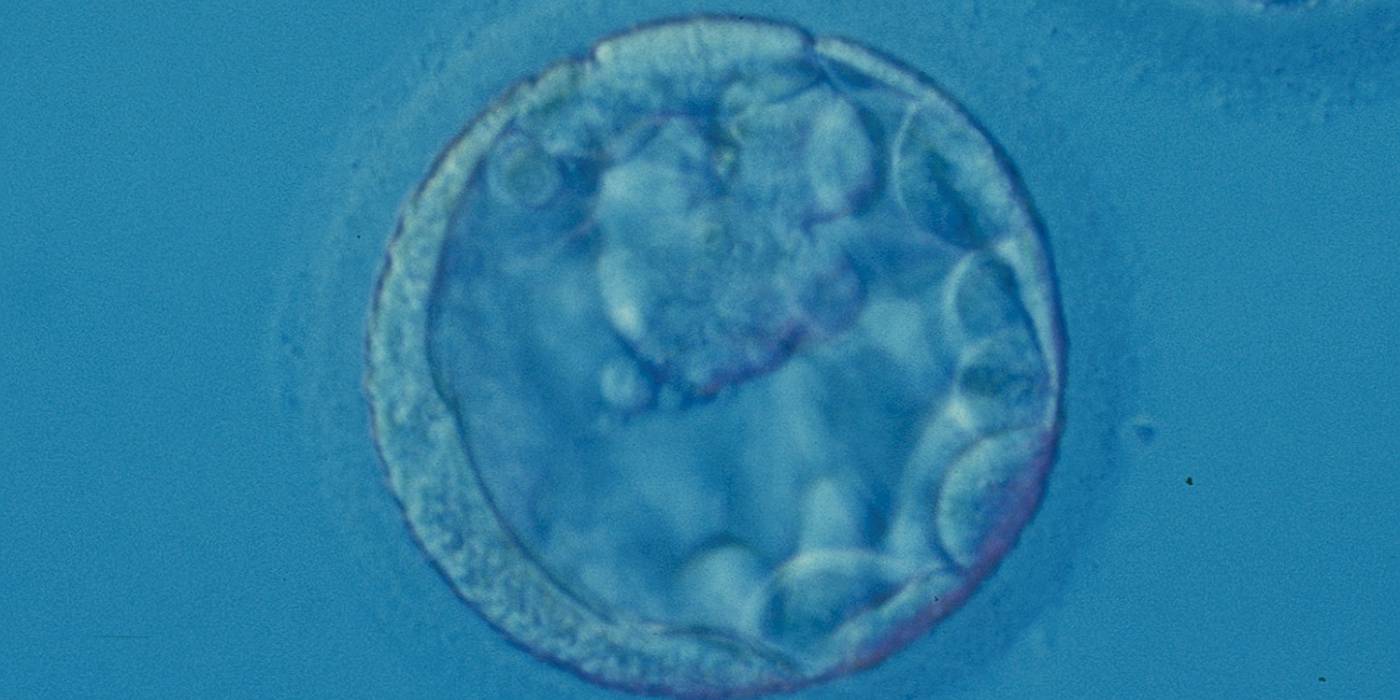
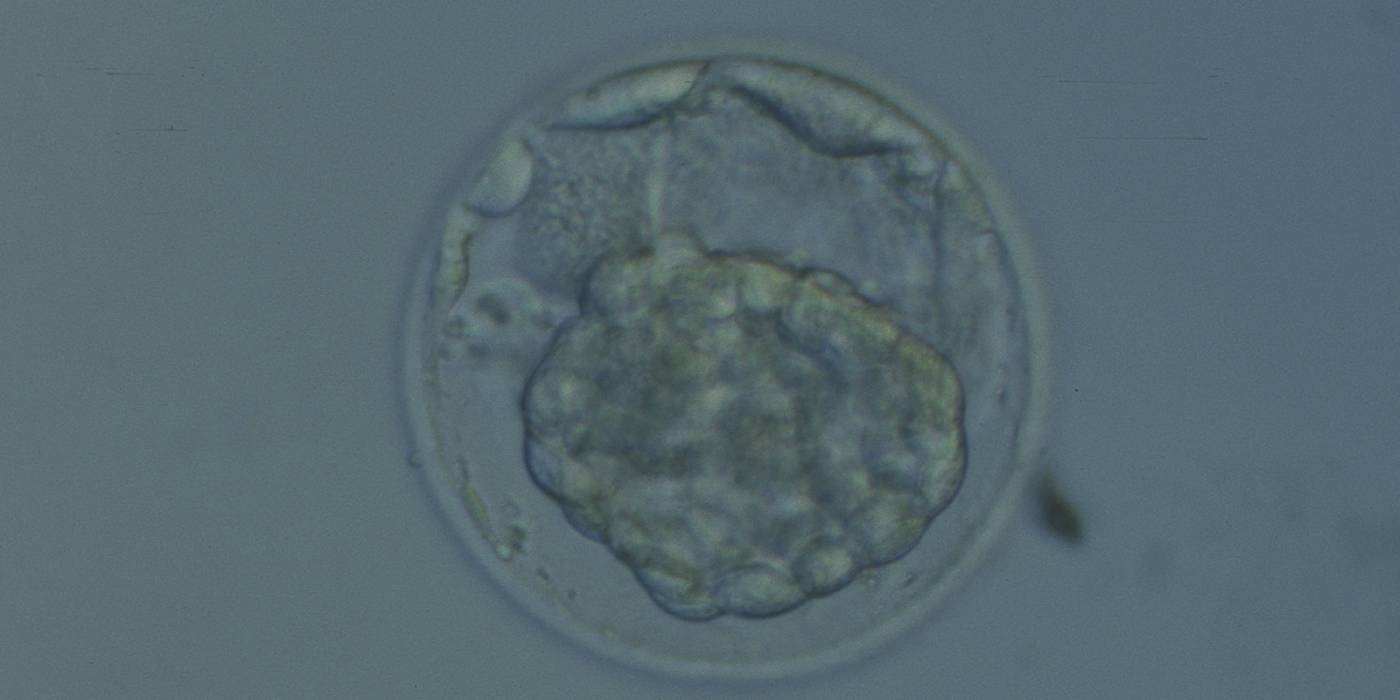
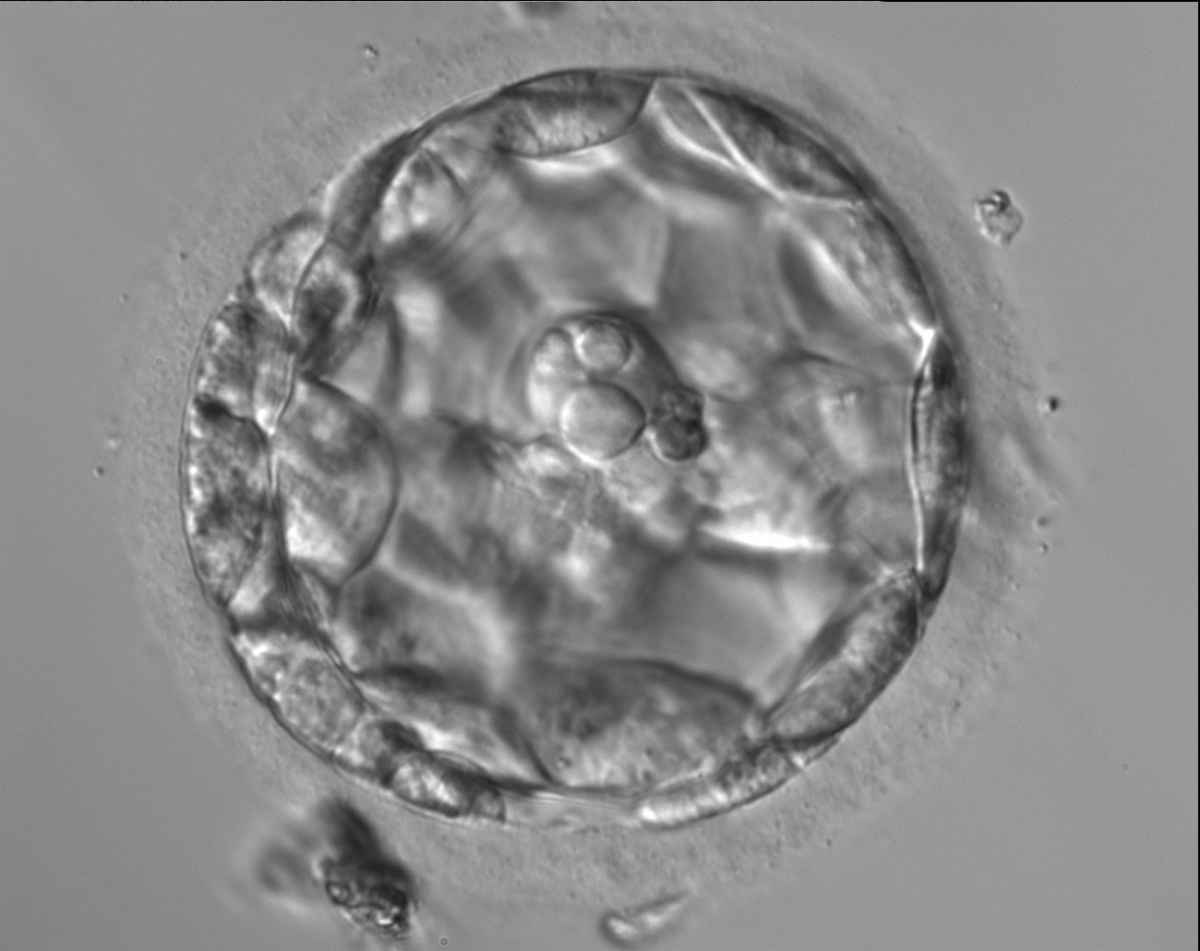
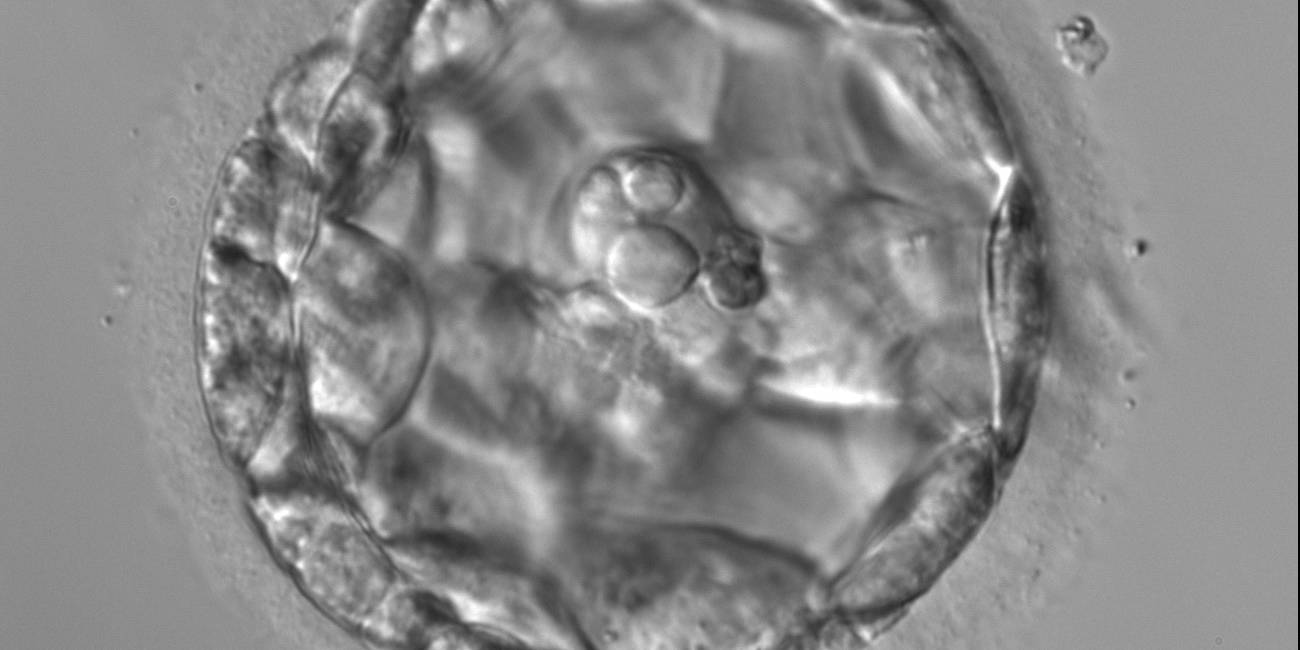
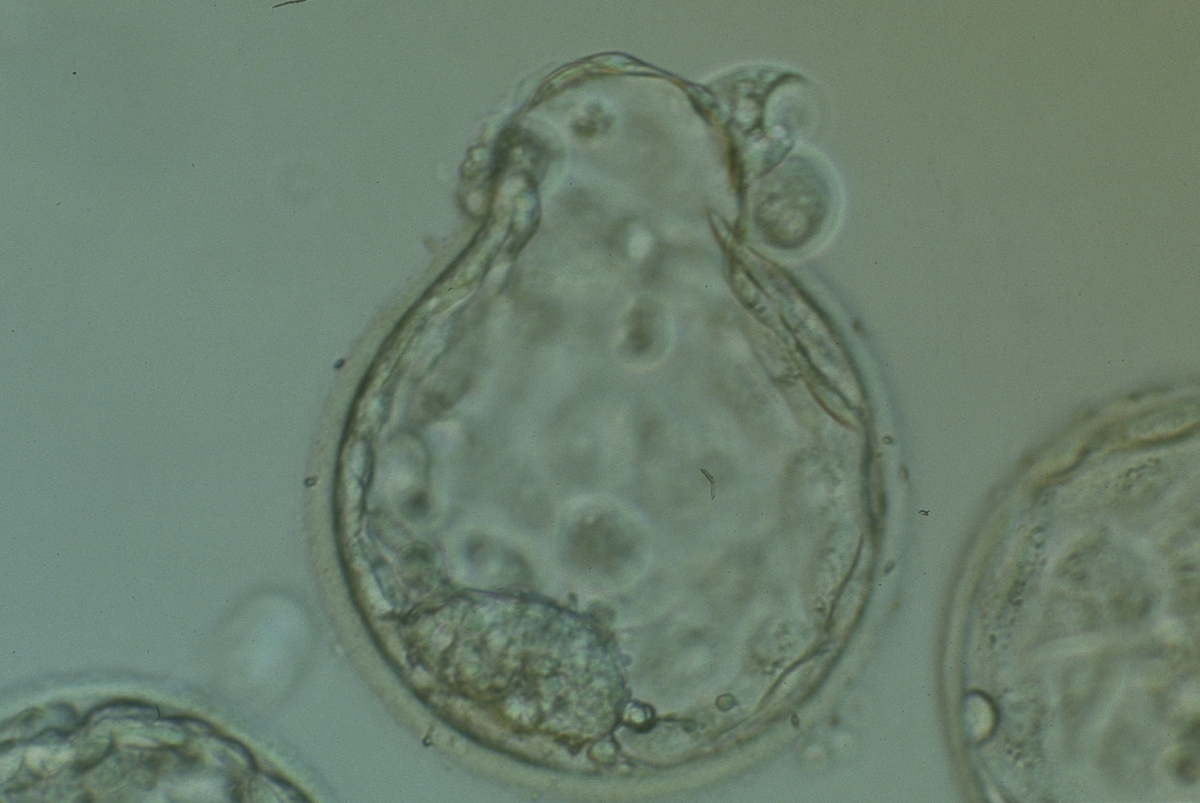
Arrested cells that have arisen at any time throughout preimplantation development are often excluded from the formation of the blastocyst and are sequestered to the PVS and can be observed between the further developing blastocyst and the ZP (Figs 383–385). Occasionally these cells, or more likely cells that have arrested much later in development, are present internally during blastocyst formation and rather than being sequestered to the PVS are sequestered to the blastocoel cavity (Figs 386–388) and take no further part in development. It has been demonstrated that excluded cells have poor gap junction communication with the embryo (Hardy et al., 1996). It was hypothesized that the presence of isolated cells in the blastocyst at a time when phagocytosis is possible indicates the absence of cell surface markers that promote their ingestion by neighboring cells (Hardy, 1997).
Kovacic et al. (2004) studied the developmental capacity of morphologically suboptimal blastocysts, classified into different categories. It was found that live birth rates after transfer of poor quality blastocysts was decreased in the following order of categories compared with the live birth rate in a control group of good quality blastocysts (45.2%): blastocysts with cytoplasmic fragments and necrotic TE (32.8%), blastocysts with a maximum of 20% excluded blastomeres (16.7%), necrotic TE and ICM (7.7%) and finally very small blastocysts with >20% excluded cells (1.2%).
Article references:
Dokras A, Sargent IL, Barlow DH. Human blastocyst grading: an indicator of developmental potential? Hum Reprod 1993;8:2119-2127.
Abstract/FREE Full Text
Hardy K. Cell death in the mammalian blastocyst. Mol Hum Reprod 1997;3:919-925.
Abstract/FREE Full Text
Hardy K. Apoptosis in the human embryo. Rev Reprod 1999;4:125-134.
Abstract
Hardy K, Handyside AH, Winston R. The human blastocyst: cell number, death and allocation during late preimplantation development in vitro. Development 1989;107:597-604.
Abstract
Hardy K, Warner A, Winston R, Becker L. Expression of intercellular junctions during preimplantation development of the human embryo. Mol Hum Reprod 1996;2:621-632.
Abstract/FREE Full Text
Kovacic B, Vlaisavljevic V, Reljic M, Cizek-Sajko M. Developmental capacity of different morphological types of day 5 human morulae and blastocysts. Reprod Biomed Online 2004;8:687-694.
CrossRef | Medline | Web of Science | Google Scholar
Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 1980;284:555-556.
CrossRef | Medline | Web of Science | Google Scholar