B. Fragmentation
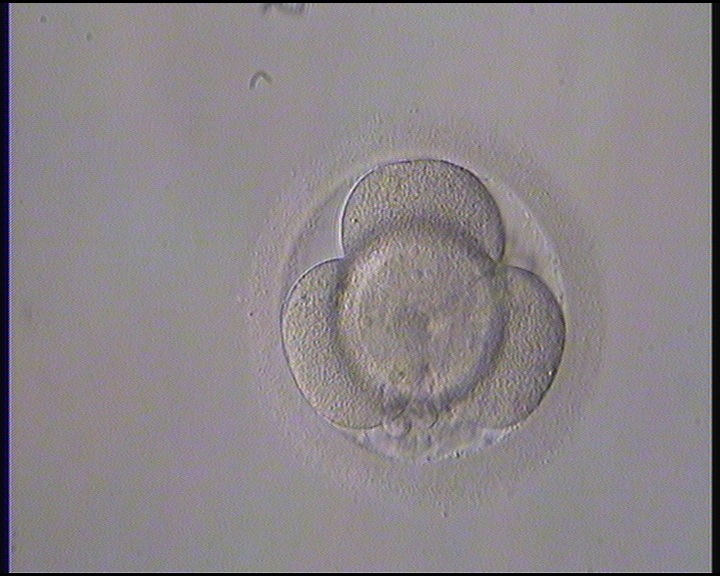
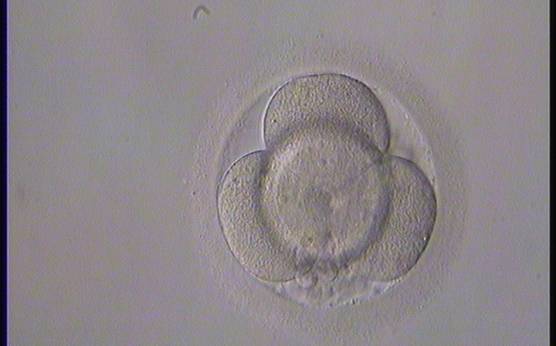
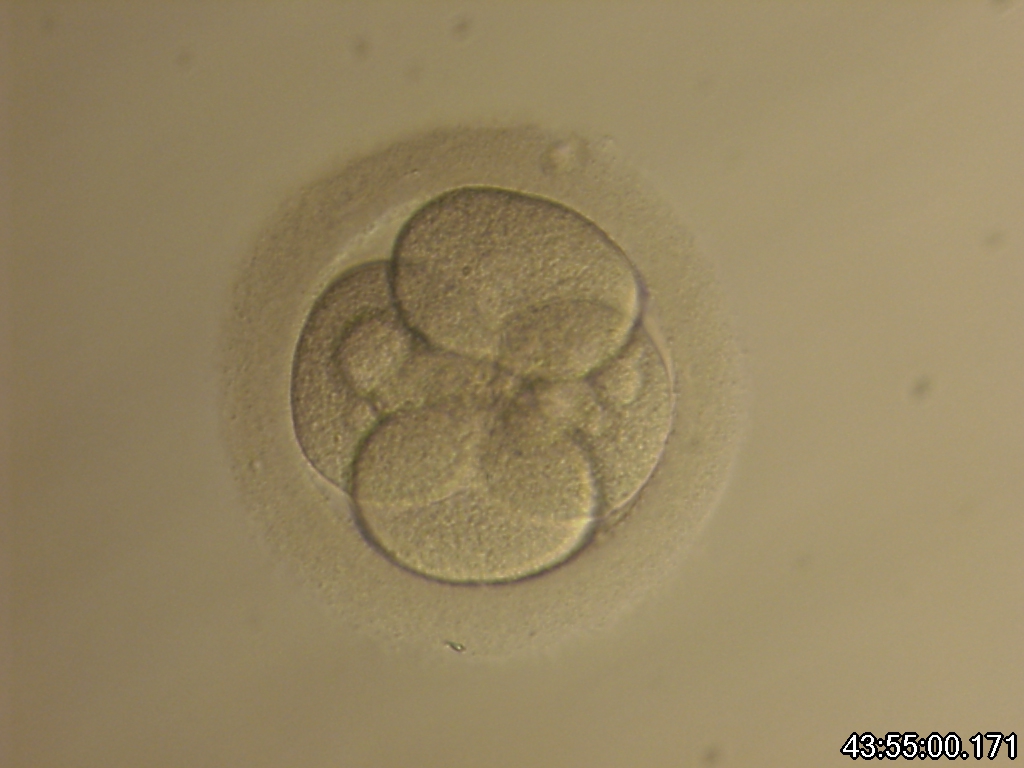
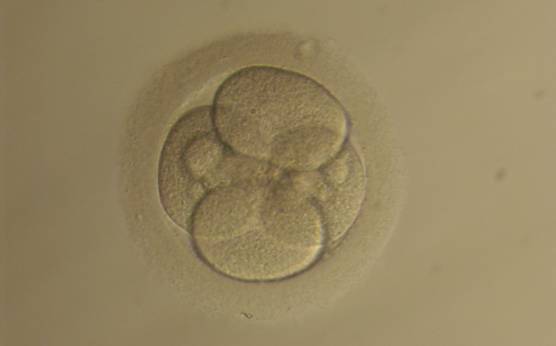
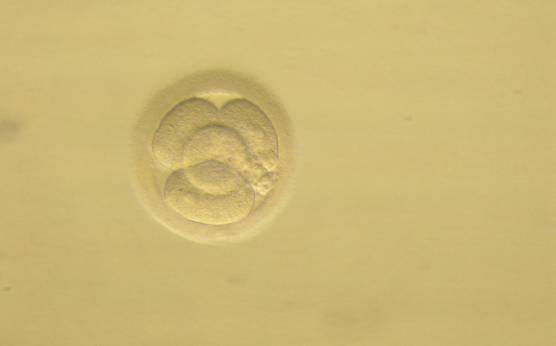
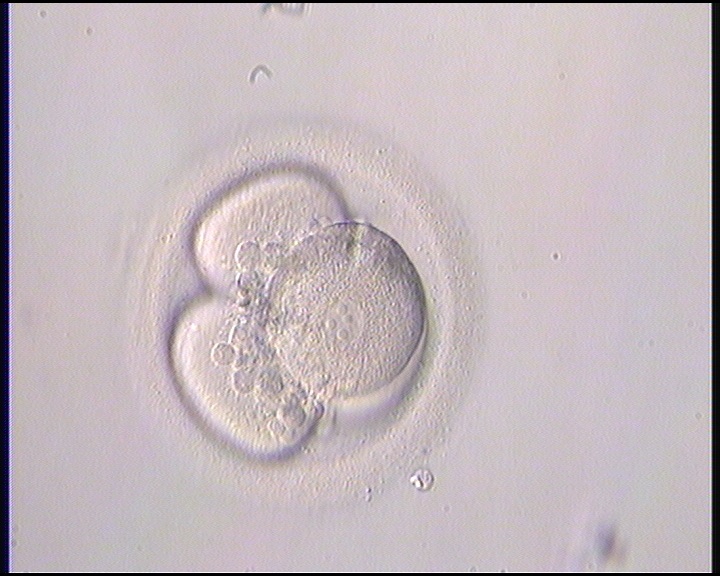
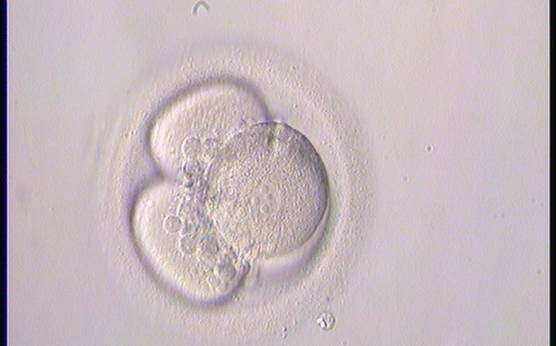
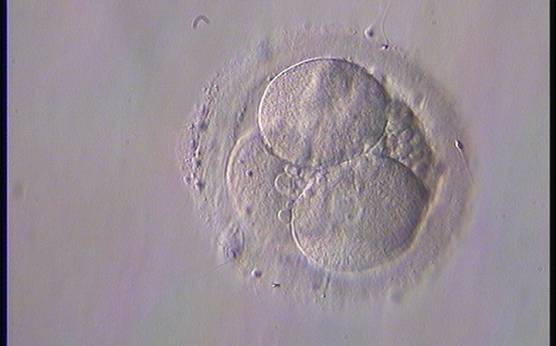
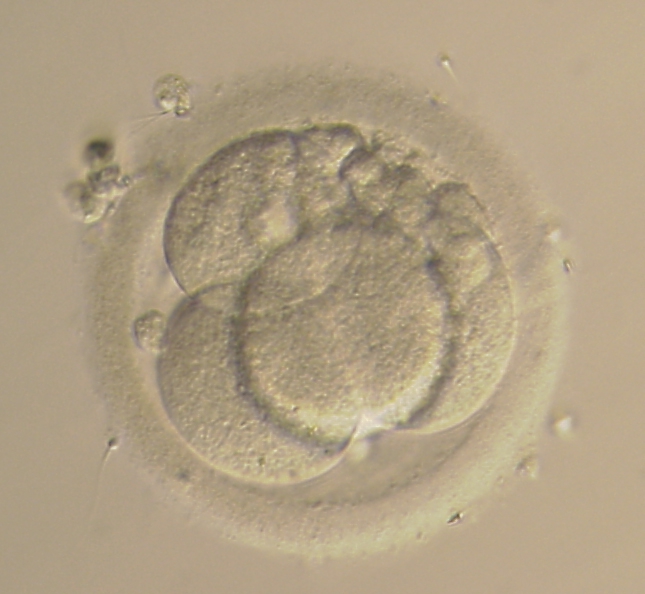
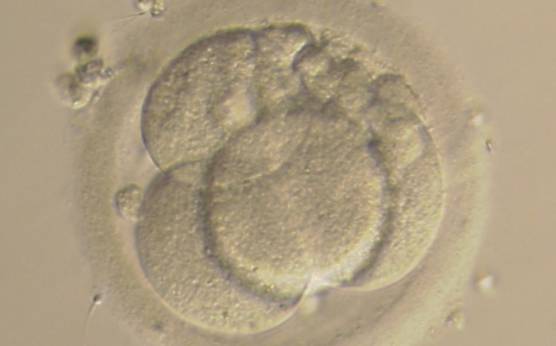
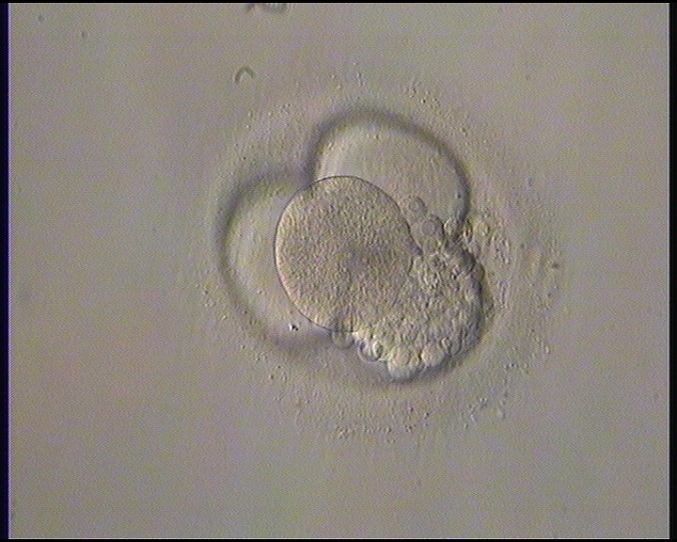
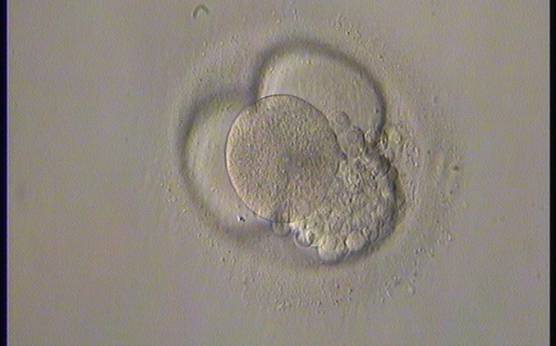
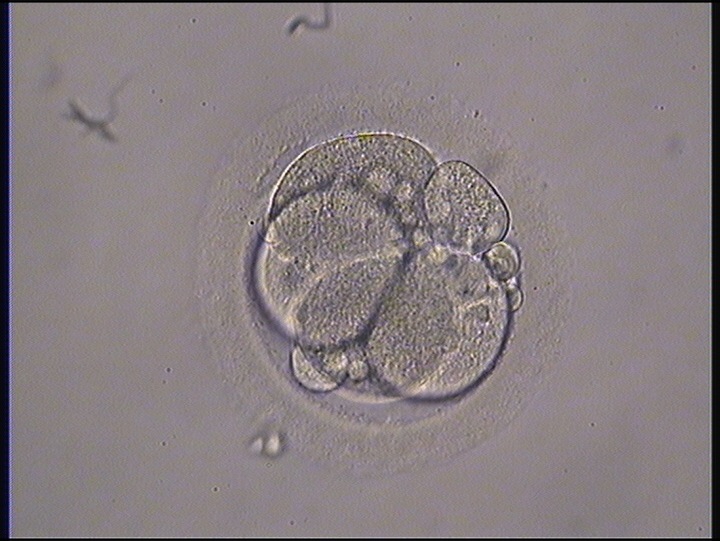
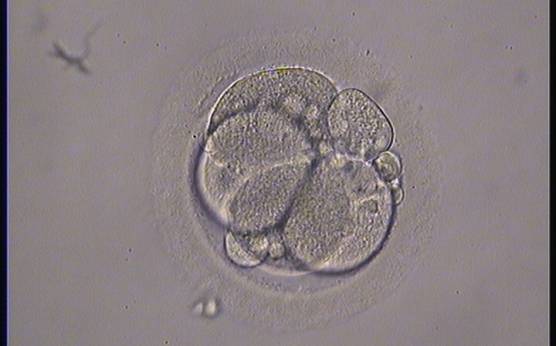
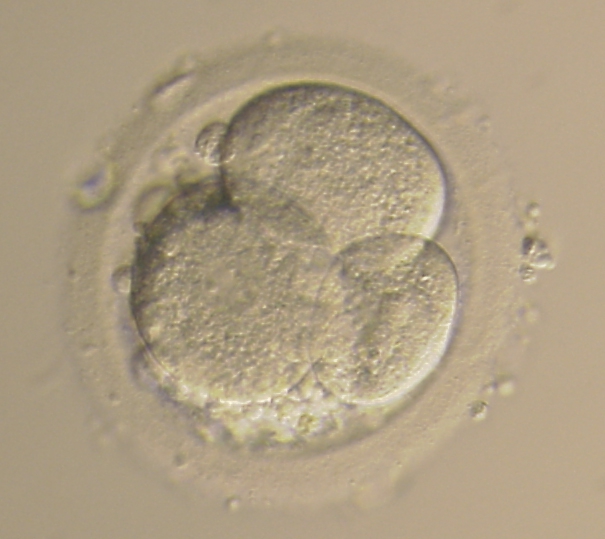
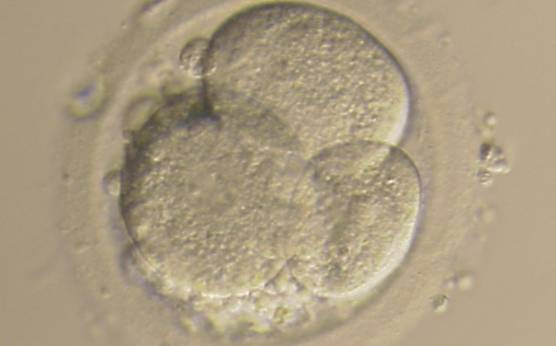
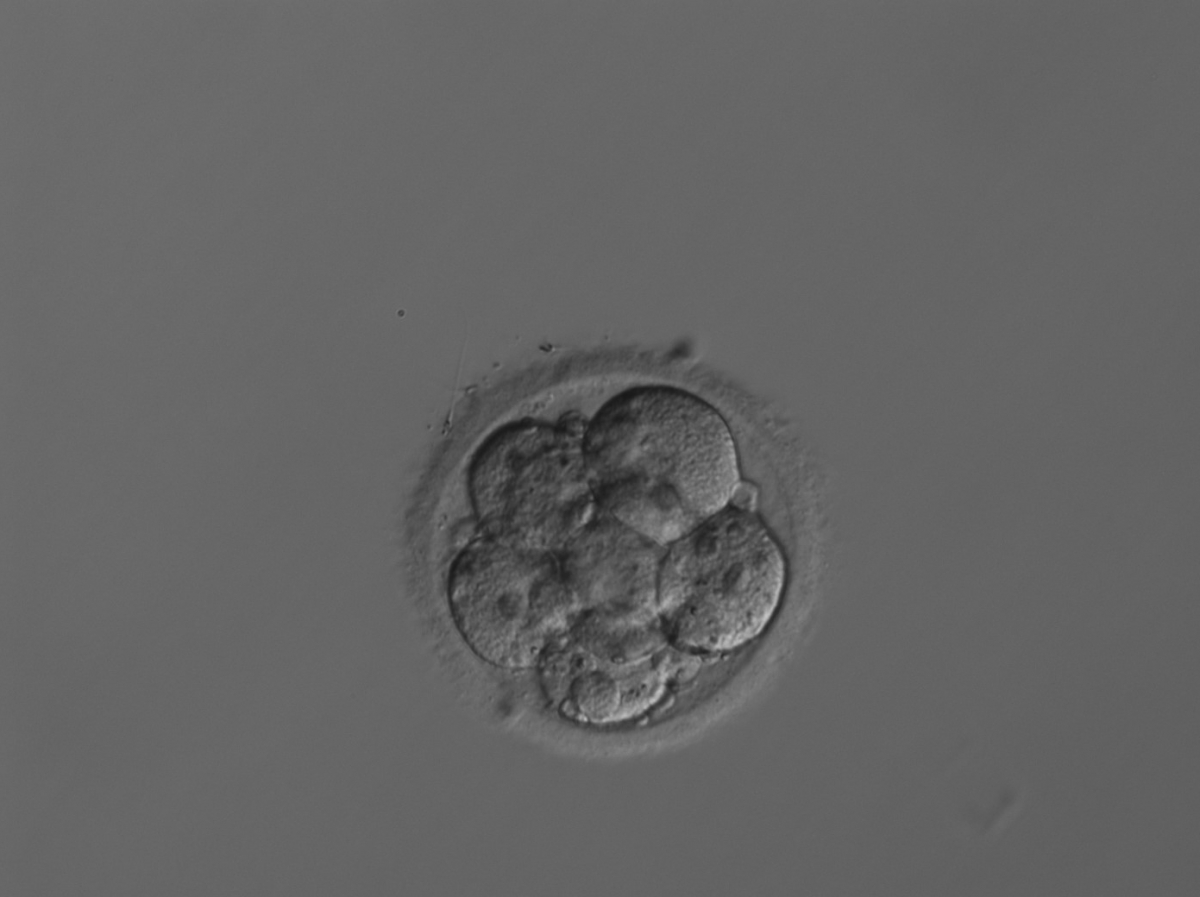
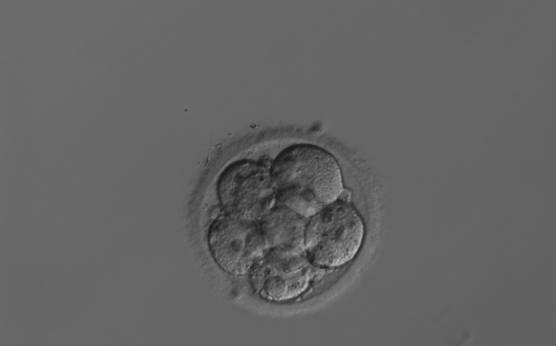
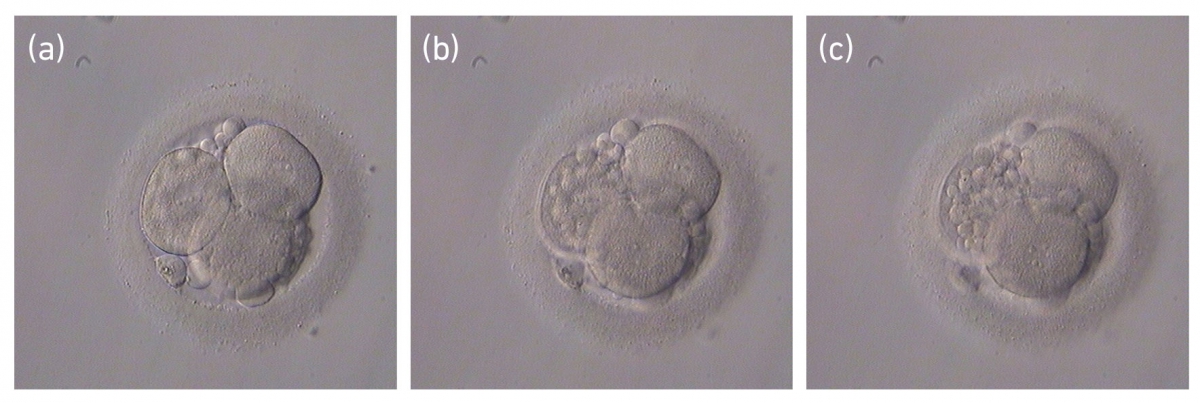
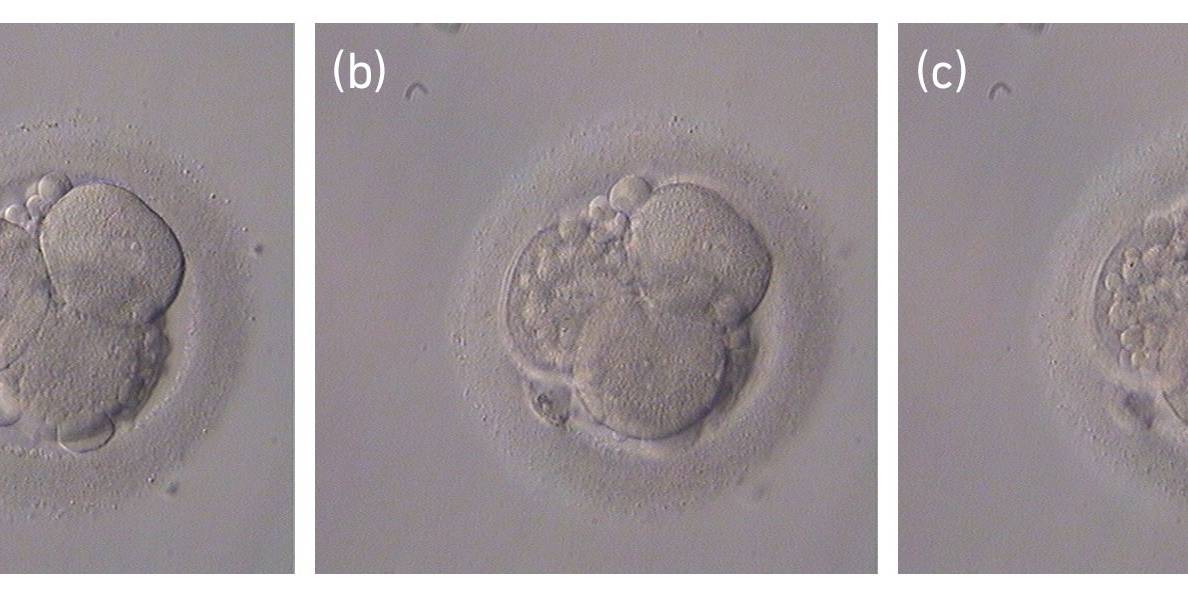
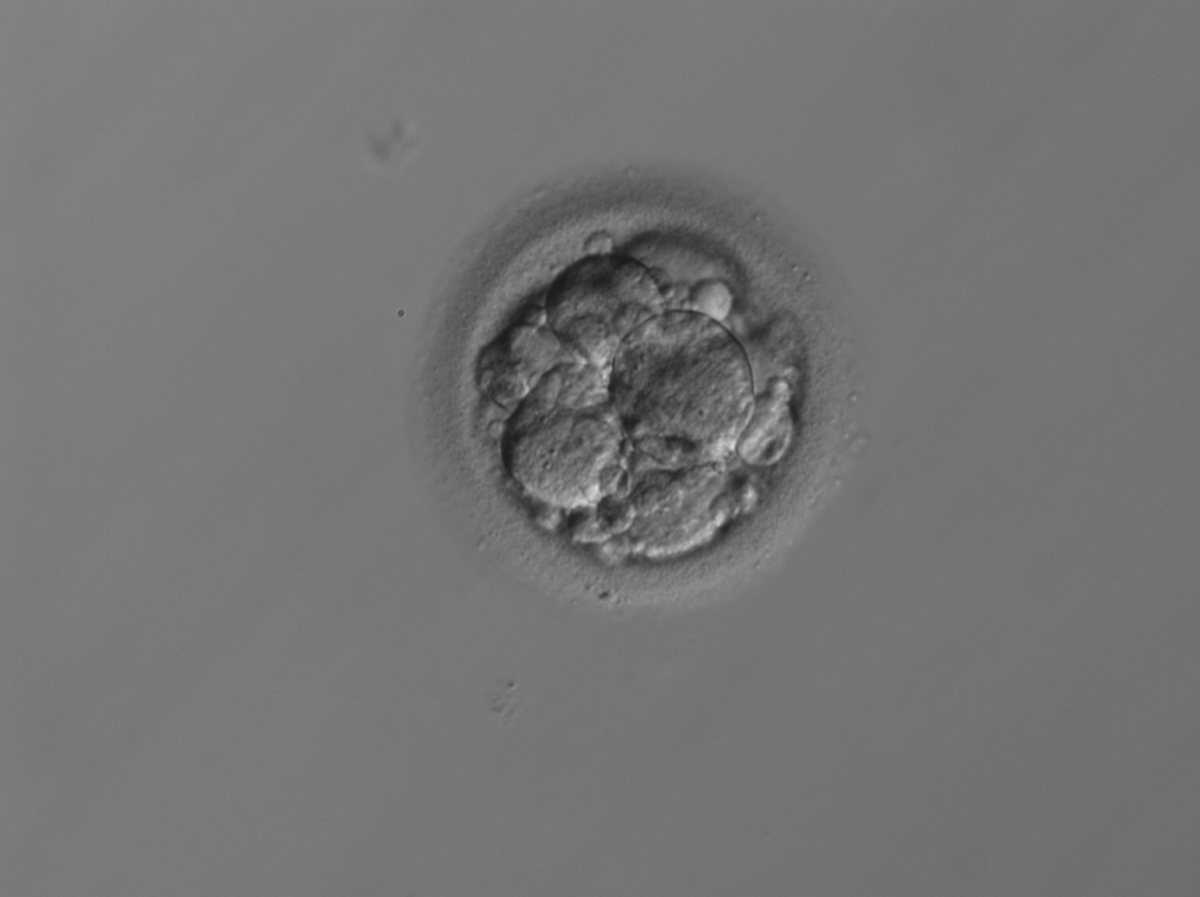
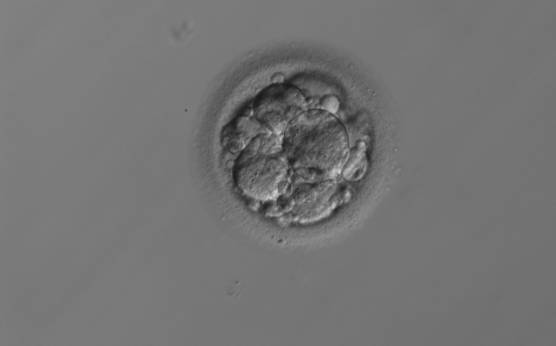
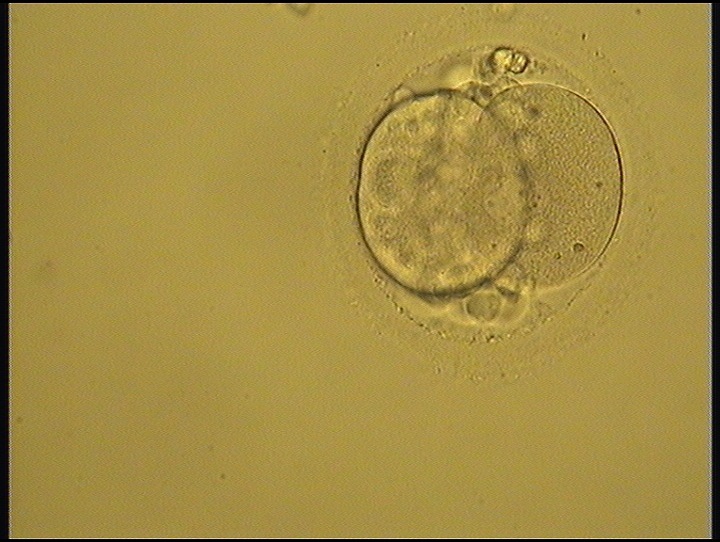
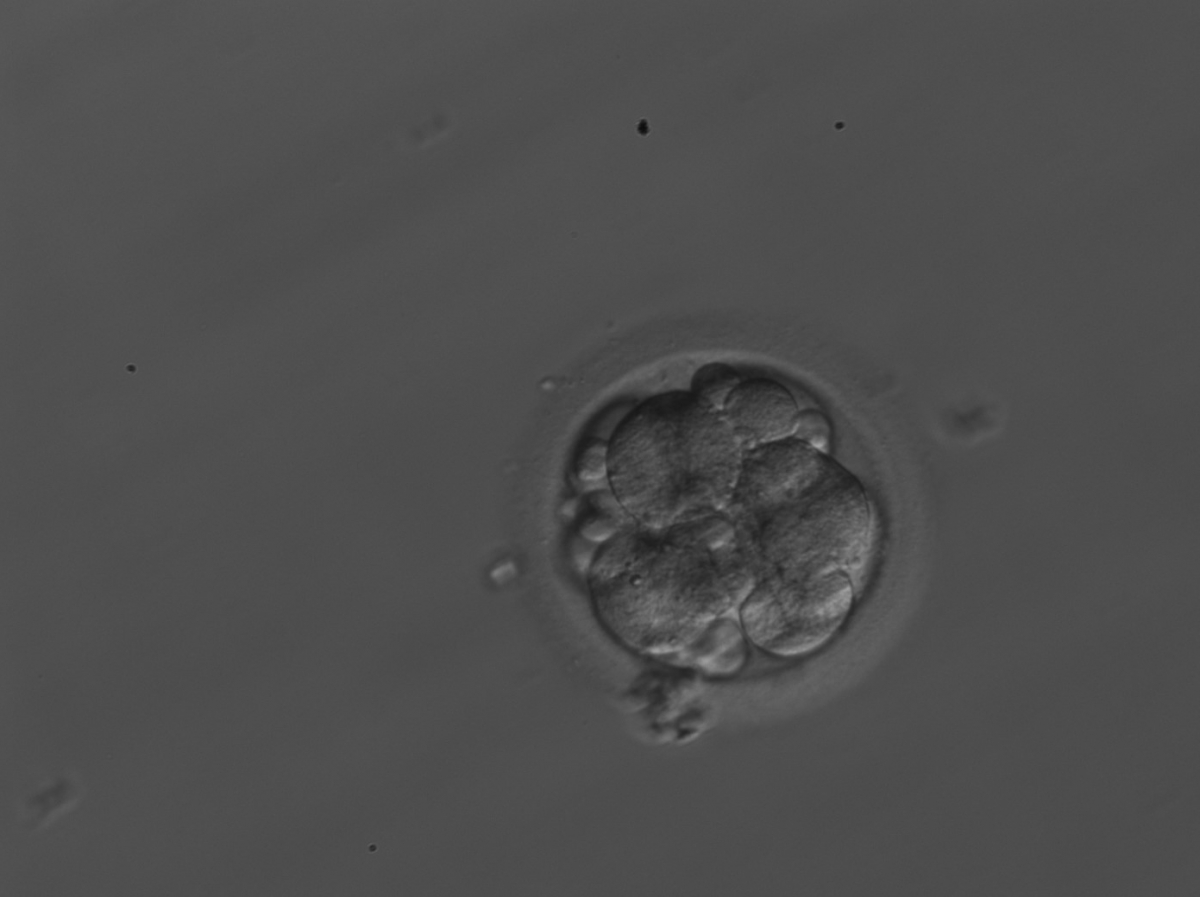
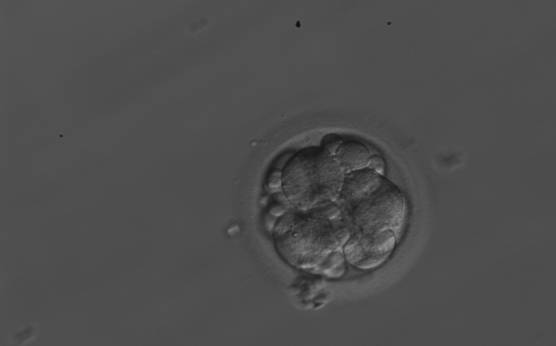
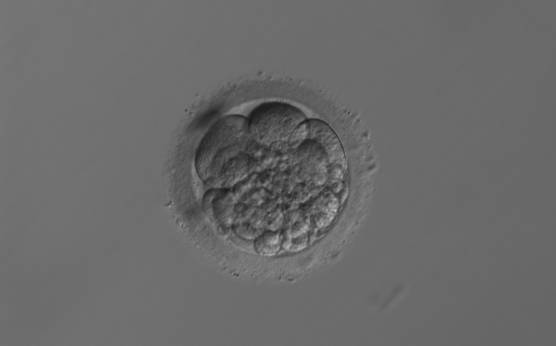
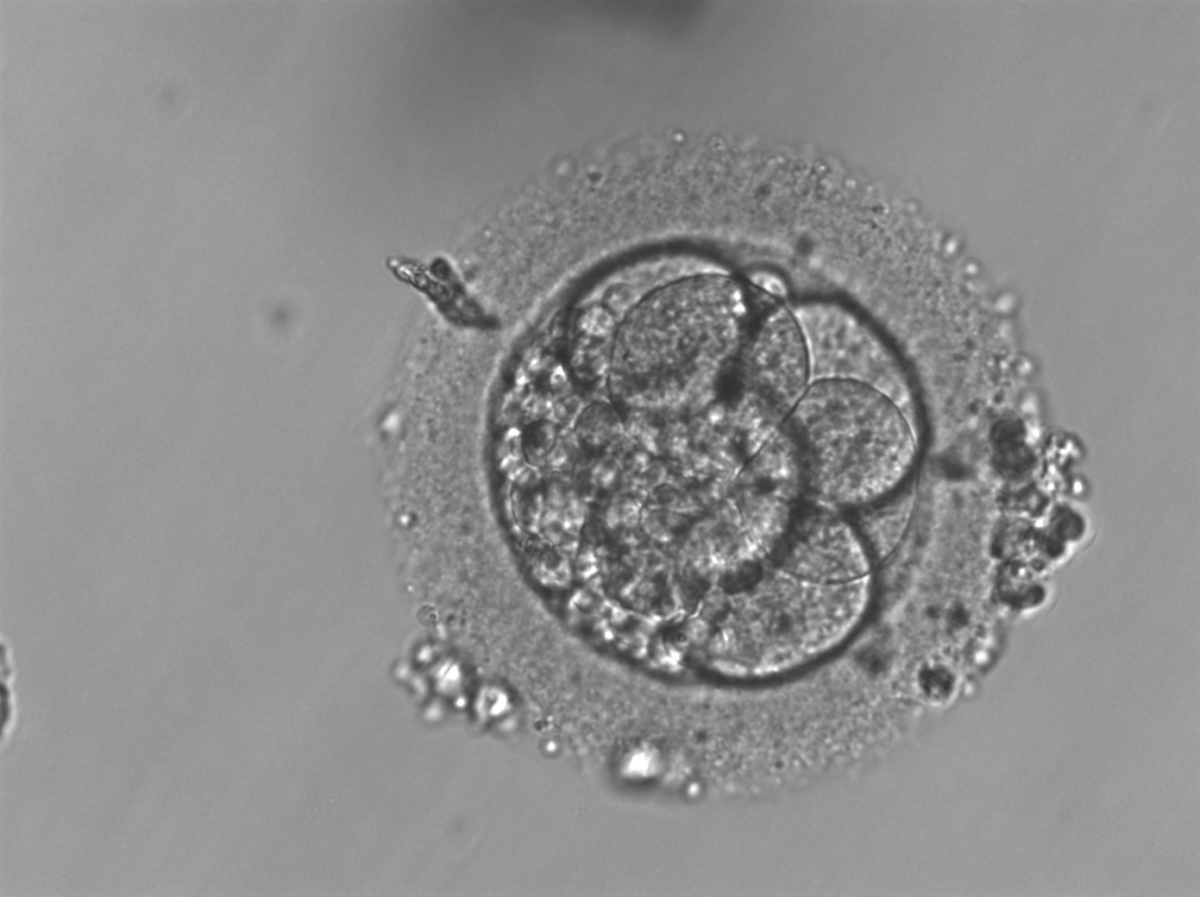
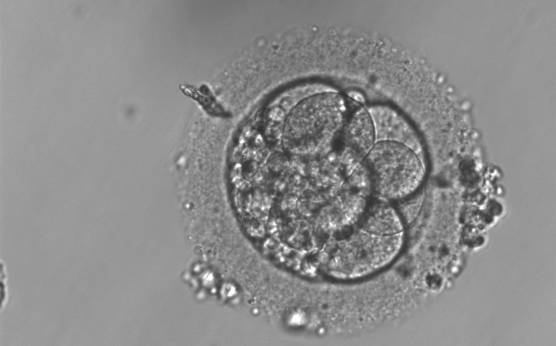
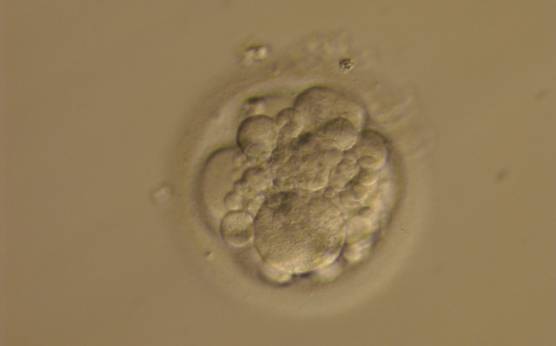
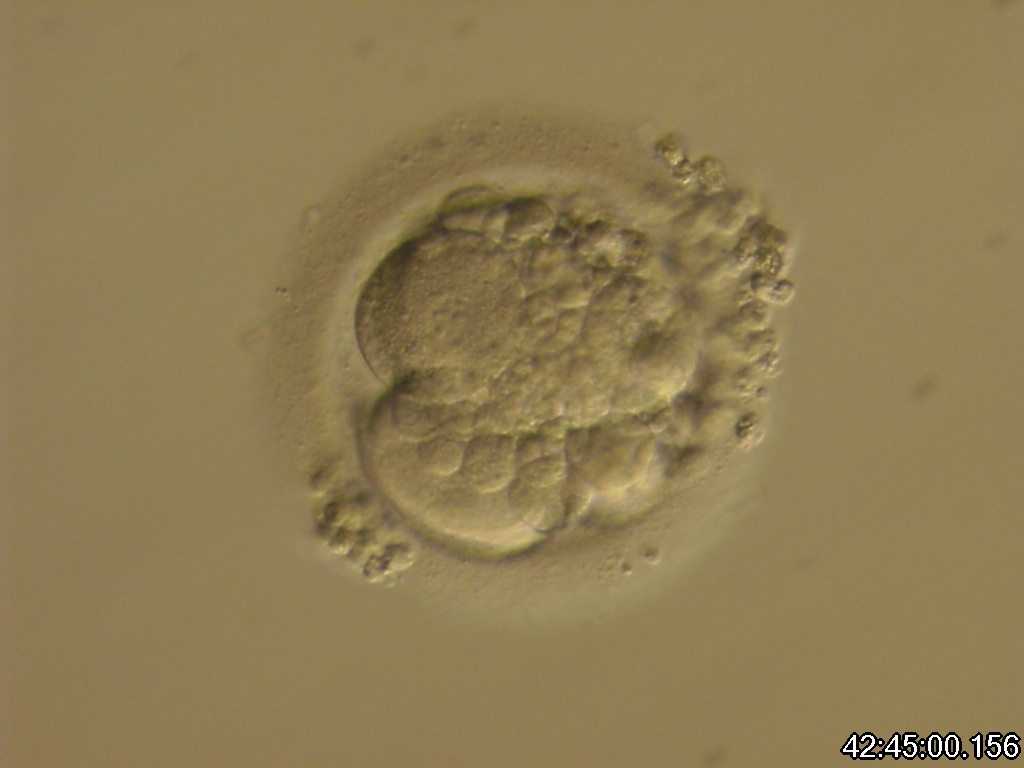
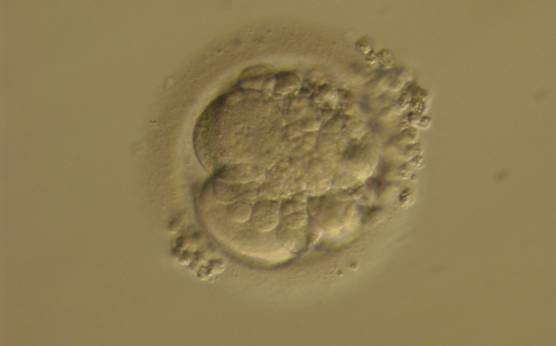
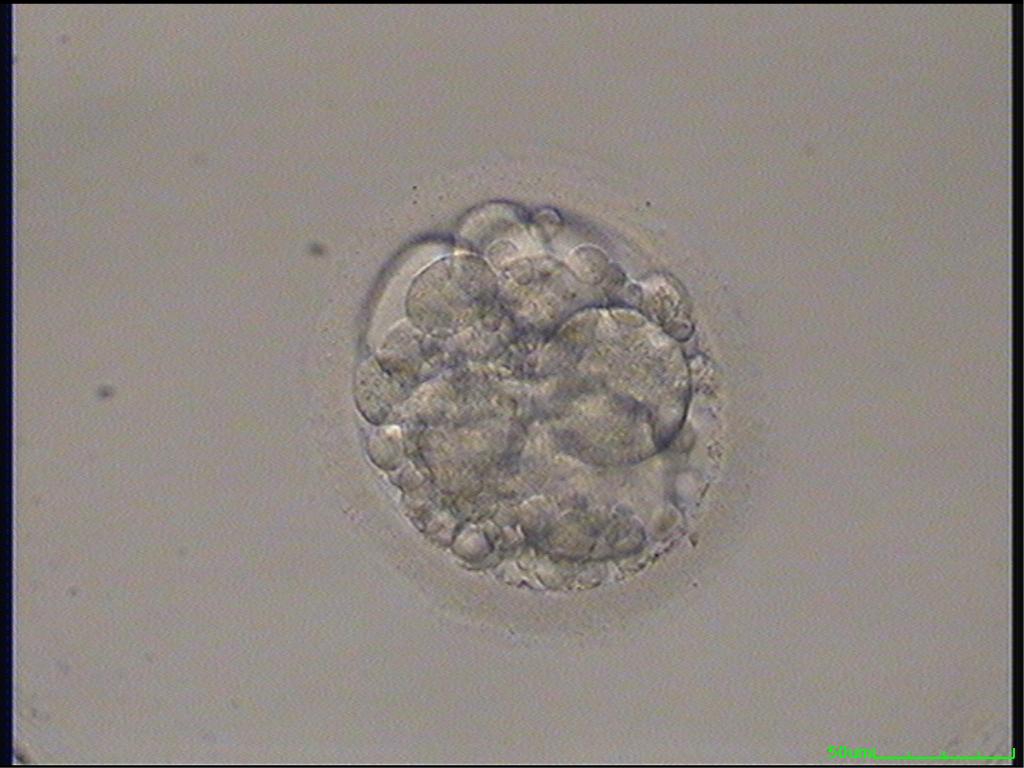
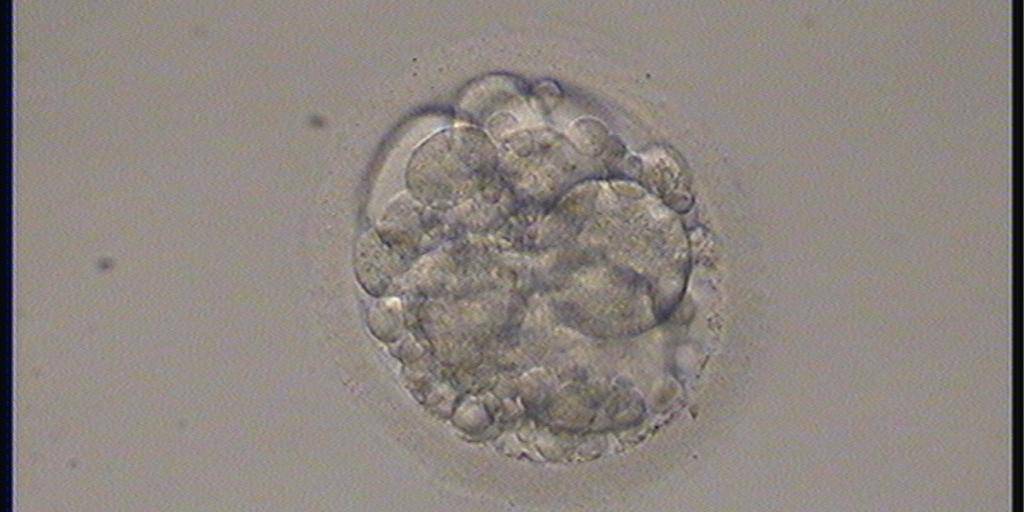
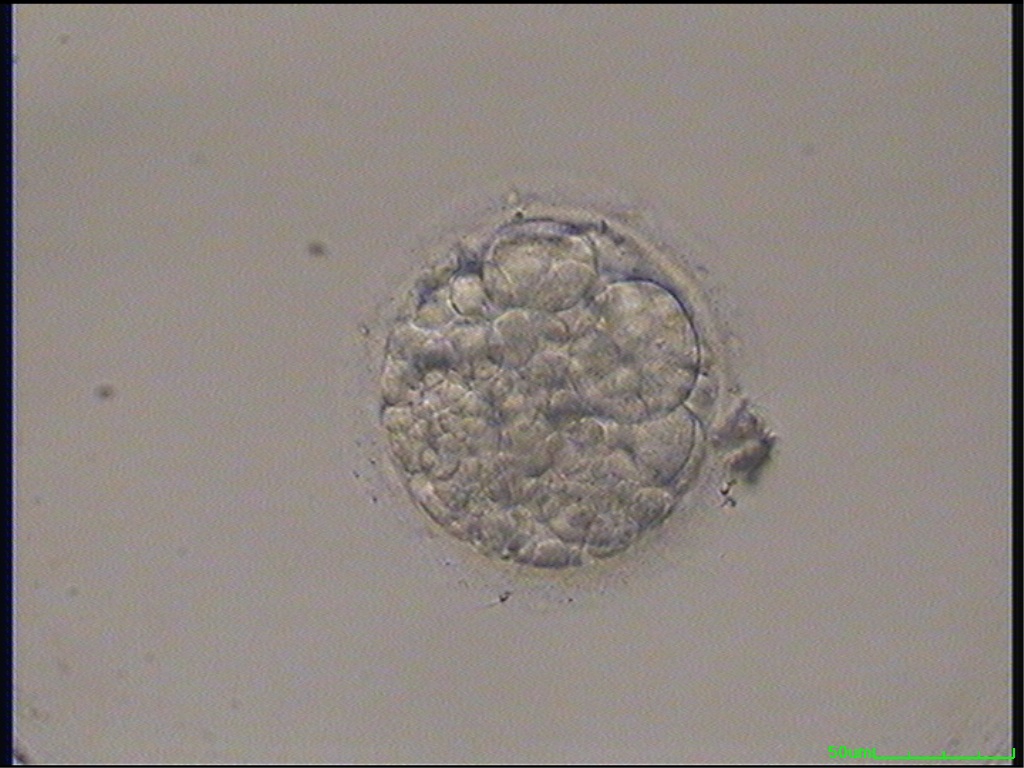
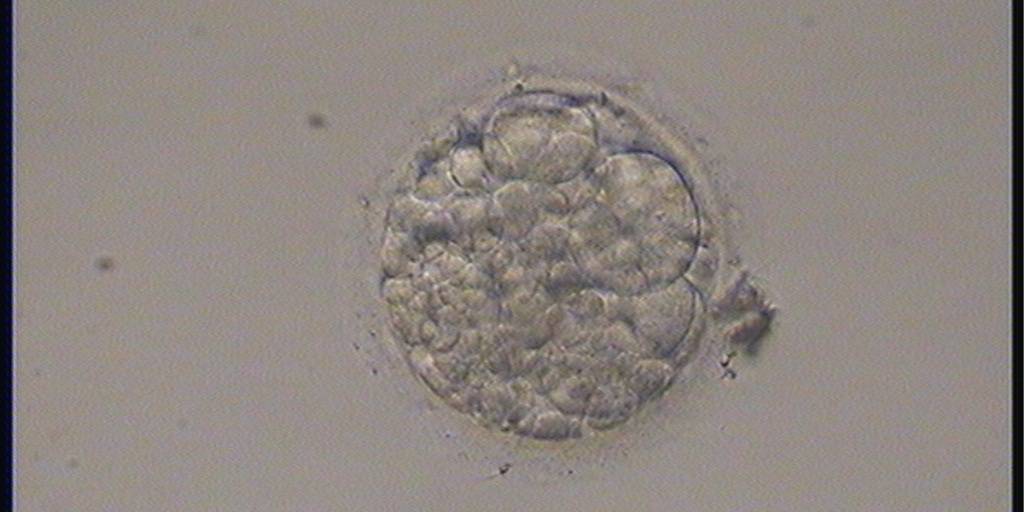
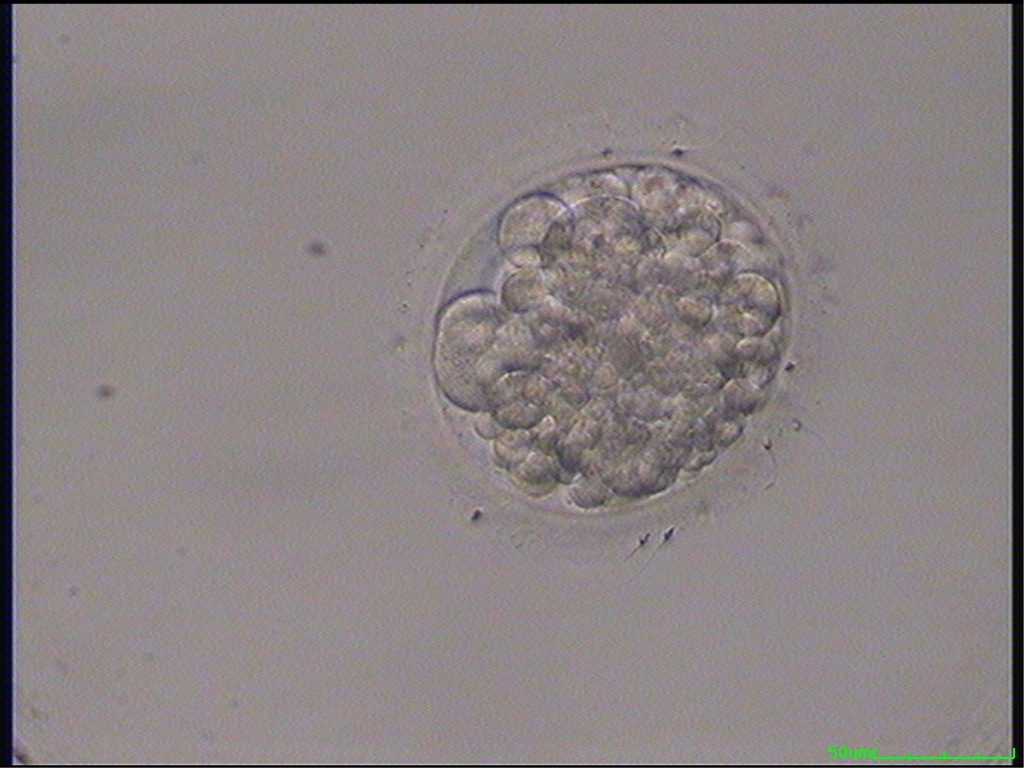
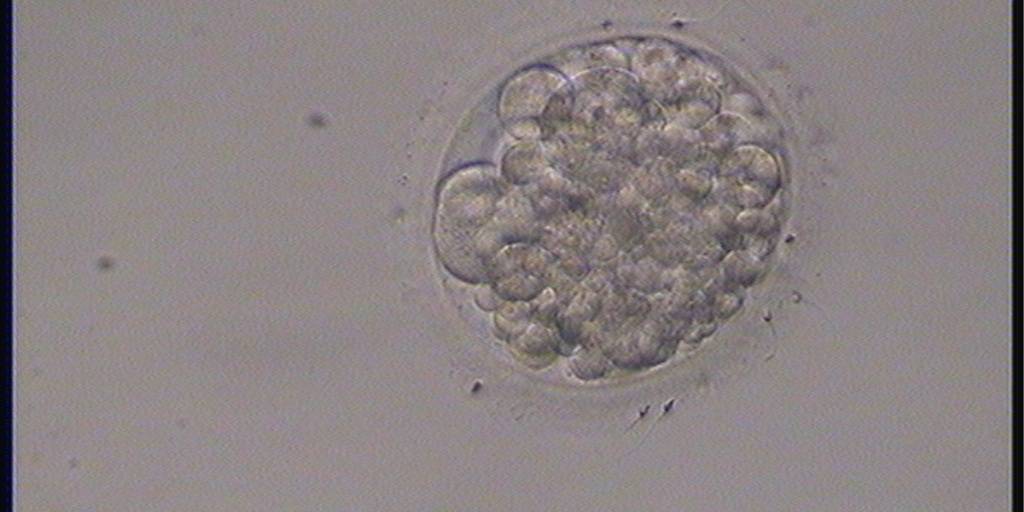
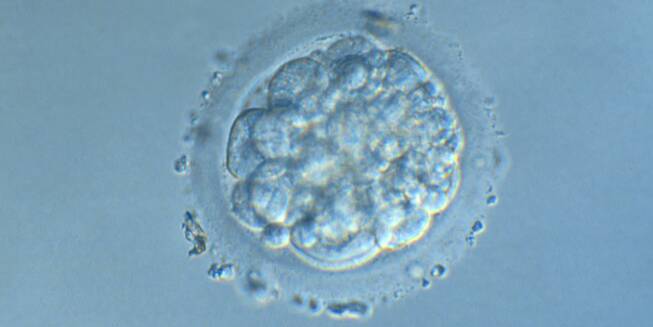
Small portions of cytoplasm enclosed by a cell membrane but usually not containing DNA are often formed during cell division. Fragmentation is therefore defined as the presence of anucleate structures of blastomeric origin (Keltz et al., 2006) and evaluation of the degree of fragmentation is included in almost every embryo scoring system. The degree of fragmentation is most often expressed as the percentage of the total cytoplasmic volume. The relative degree of fragmentation is defined as mild (<10%, Figs 223–225), moderate (10–25%, Figs 226–235) and severe (>25%, Figs 236–242).
It is often difficult to make the distinction between a large anucleate fragment and a small (nucleated) cell. Johansson et al. (2003) showed that portions of cytoplasm that were <45 µm in diameter on Day 2 and <40 µm in diameter on Day 3 did not contain DNA, and the authors suggested a standardization of defining fragments as all structures below these sizes.
It has been shown that a high degree of fragmentation correlates negatively with implantation and pregnancy rates (Racowsky et al., 2000), while the presence of minor amounts of fragmentation has no negative or possibly even a positive impact (Alikani et al., 1999). Two distinctly different types of fragmentation have been documented by time-lapse analysis in human embryos: definitive fragmentation, characterized as stable persistent fragments clearly detached from blastomeres and pseudo-fragmentation, characterized by a transient appearance during, or shortly after, cell cleavage, but not detected during later development (Van Blerkom et al., 2001).
Increasing fragmentation also results in reduced blastocyst formation and can influence allocation of cells during differentiation (Hardy et al., 2003). The spatial distribution of the fragments in the perivitelline space (PVS) can be differentiated into two patterns, i.e. scattered (Figs 224, 226, 227, 230–236, 239 and 240) or concentrated (Figs 223, 225, 228, 229, 237 and 238). The scattered appearance was found to be correlated with an increased incidence of chromosomal abnormality (Magli et al., 2007). The higher the degree of fragmentation, the more difficult it is to differentiate between scattered and concentrated fragmentation (Figs 241 and 242). Fragmentation is considered to be an essential parameter to include in the evaluation of developing embryos, as embryos with very strong and persistent fragmentation are less likely to be viable.
Article references:
Alikani M, Cohen J, Tomkin G, Garrisi GJ, Mack C, Scott RT. Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertil Steril 1999;71:836-842.
CrossRef | Medline | Web of Science | Google Scholar
Hardy K, Stark J, Winston RML. Maintenance of the inner cell mass in human blastocysts from fragmented embryos. Biol Reprod 2003;68:1165-1169.
Abstract/FREE Full Text
Johansson M, Hardarson T, Lundin K. There is a cut off limit in diameter between a blastomere and a small anucleate fragment. J Assist Reprod Genet 2003;20:309-313.
CrossRef | Medline | Web of Science | Google Scholar
Keltz MD, Shorupski JC, Bradlye K, Stein D. Predictors of embryo fragmentation and outcome after fragment removal in in vitro fertilization. Fertil Steril 2006;86:321-324.
CrossRef | Medline | Web of Science | Google Scholar
Magli MC, Gianaroli L, Ferraretti AP, Lappi M, Ruberti A, Farfalli V. Embryo morphology and development are dependent on the chromosomal complement. Fertil Steril 2007;87:534-540.
CrossRef | Medline | Web of Science | Google Scholar
Racowsky C, Jackson KV, Cekleniak NA, Fox JH, Hornstein MD, Ginsburg ES. The number of eight-cell embryos is a key determinant for selecting day 3 or day 5 transfer. Fertil Steril 2000;73:558-564.
CrossRef | Medline | Web of Science | Google Scholar
Van Blerkom J, Davis P, Alexander S. A microscopic and biochemical study of fragmentation phenotypes in stage-appropriate human embryos. Hum Reprod 2001;16:719-729.
Abstract/FREE Full Text