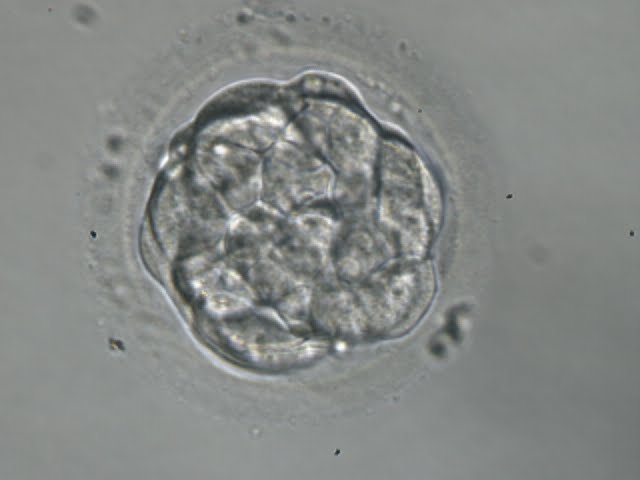
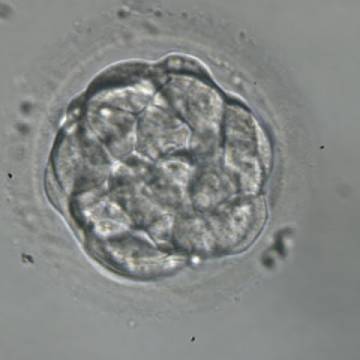
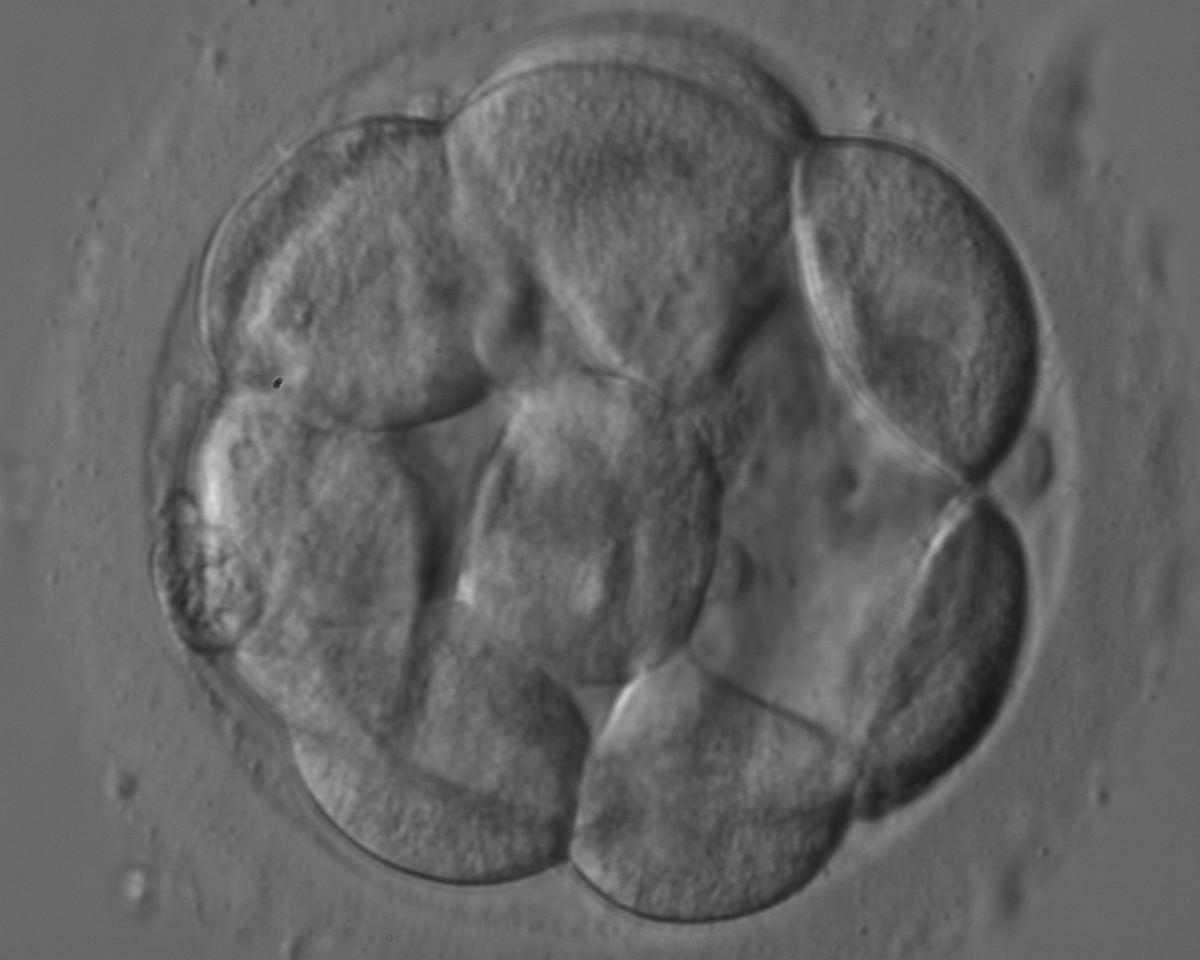
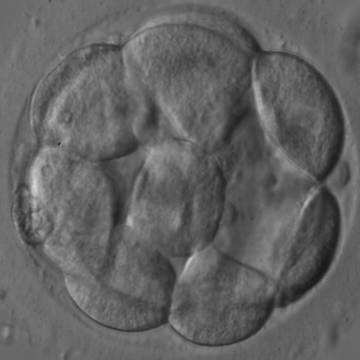
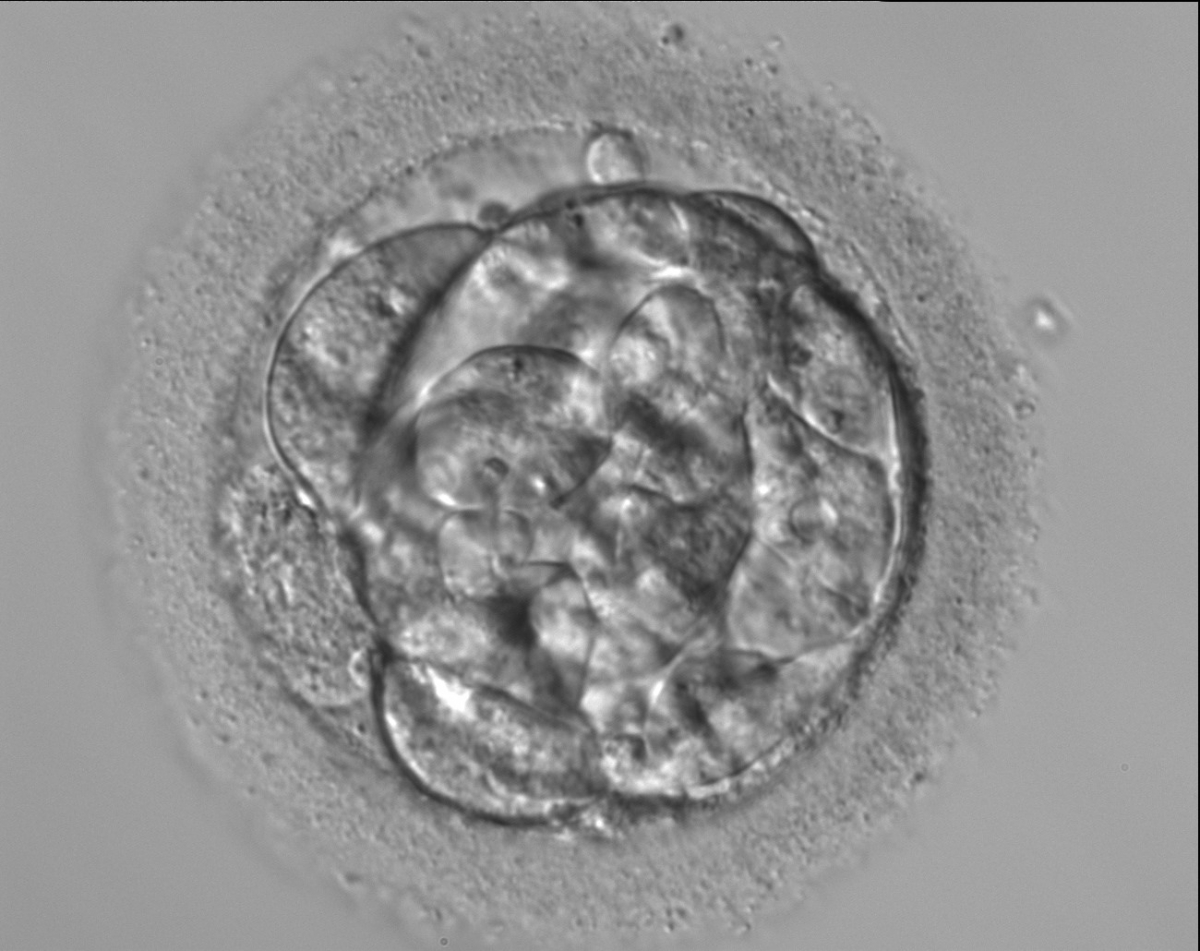
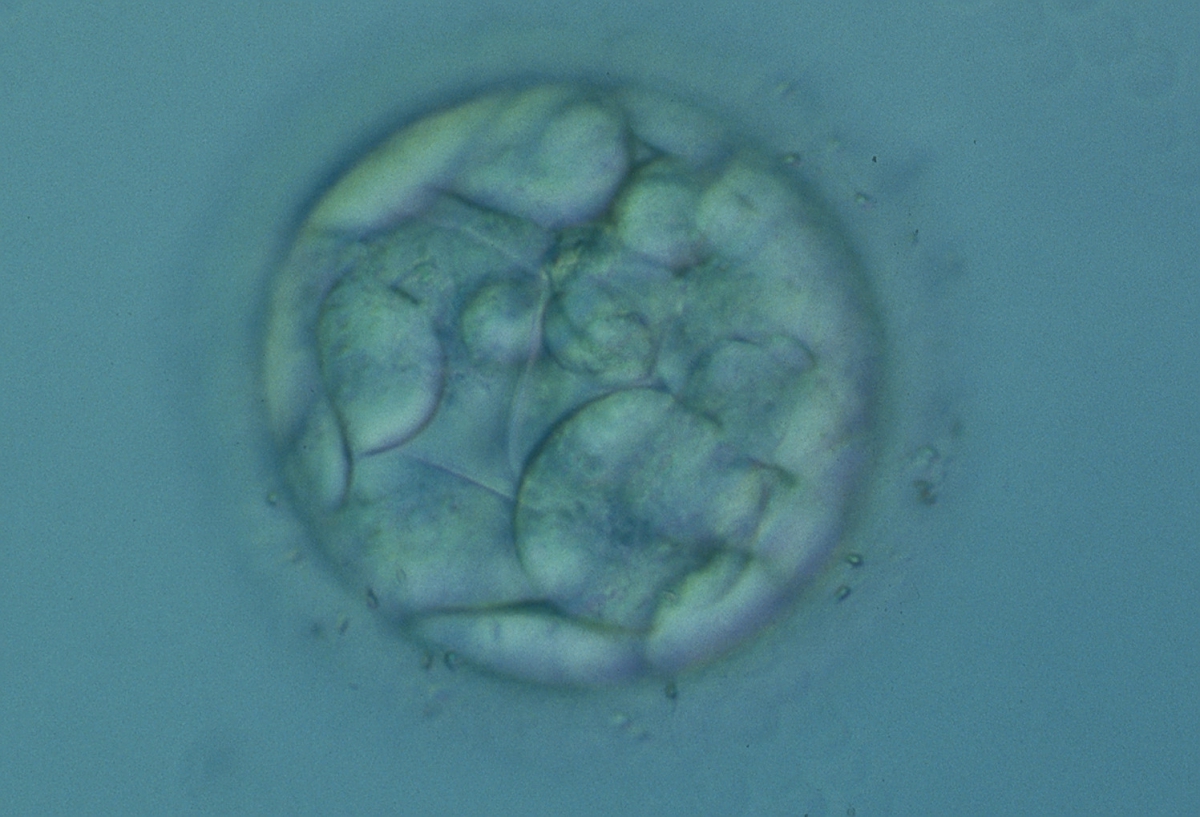
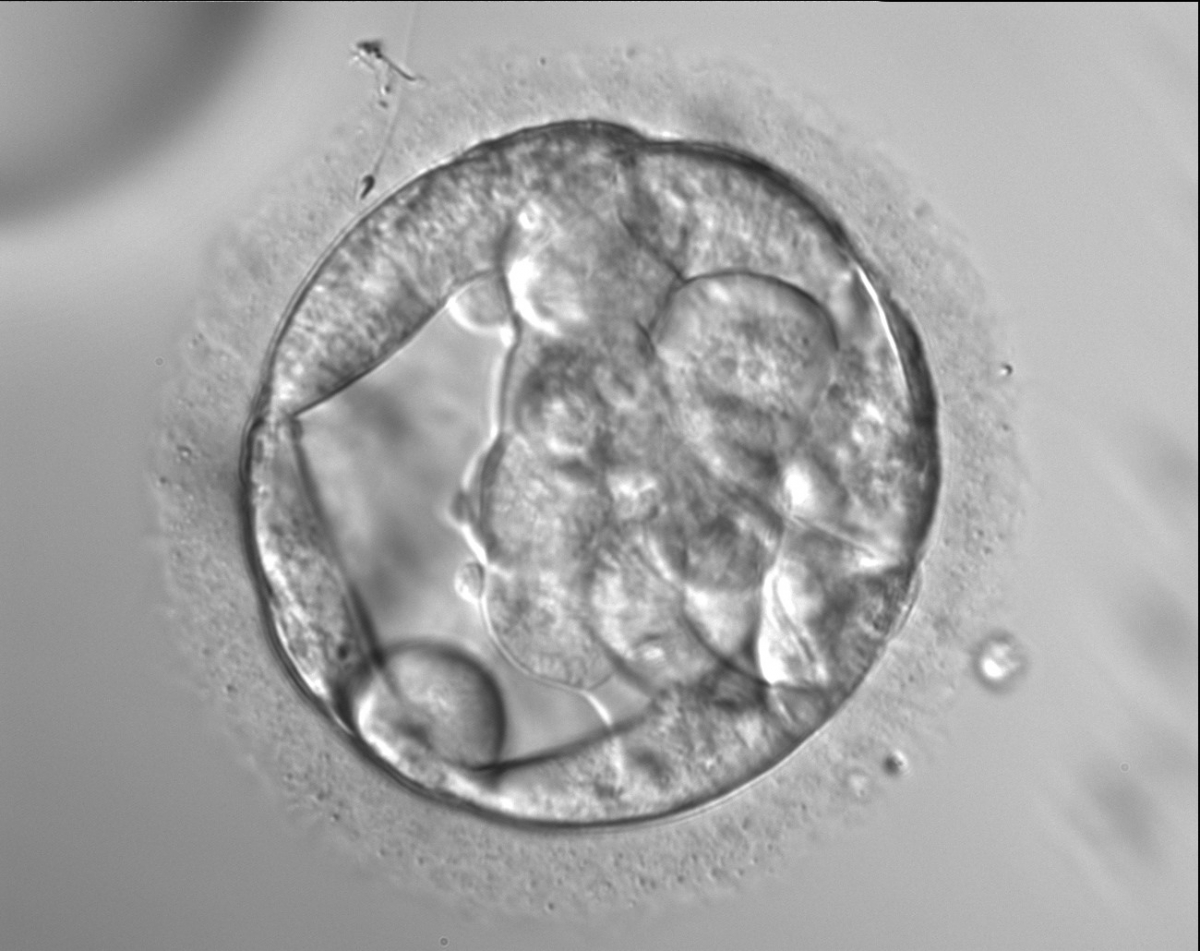
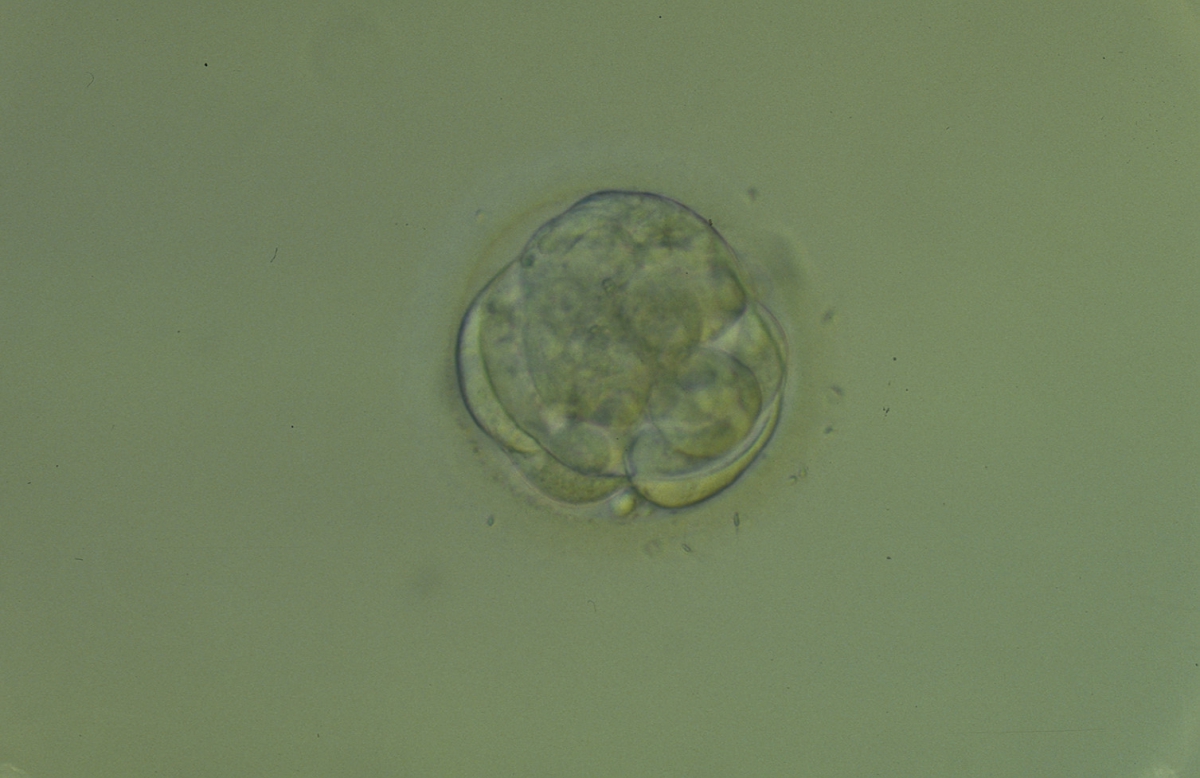
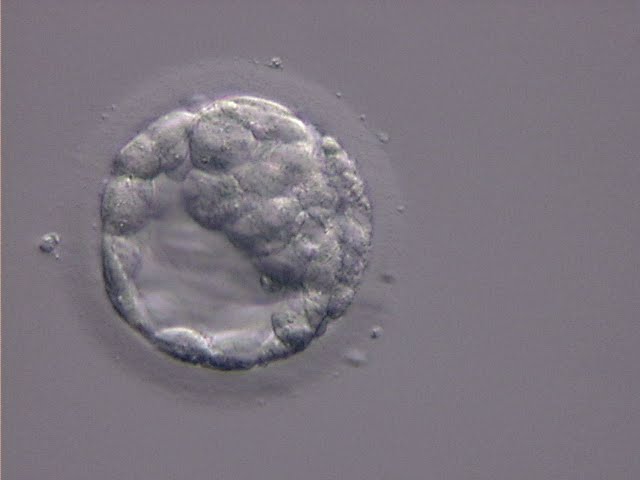
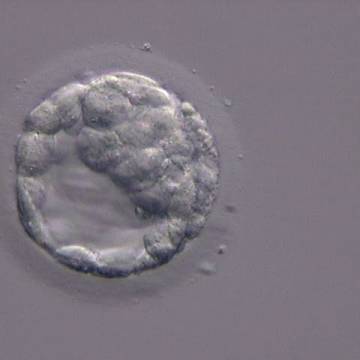
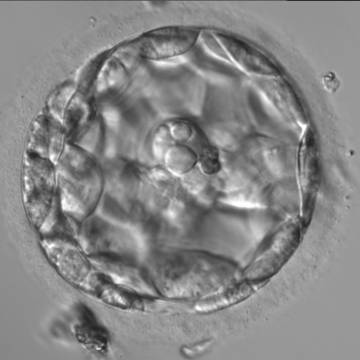
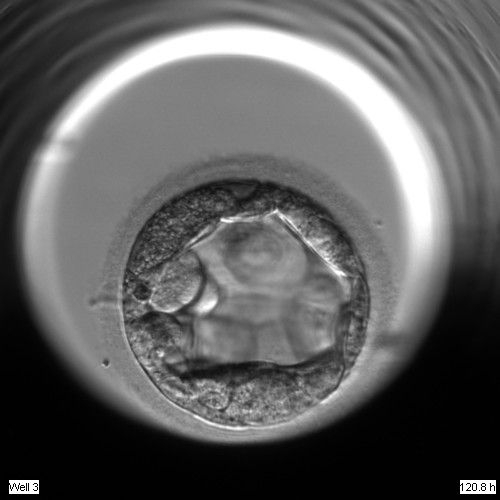
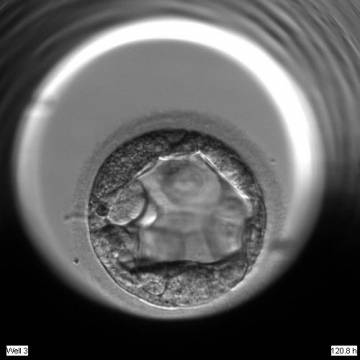
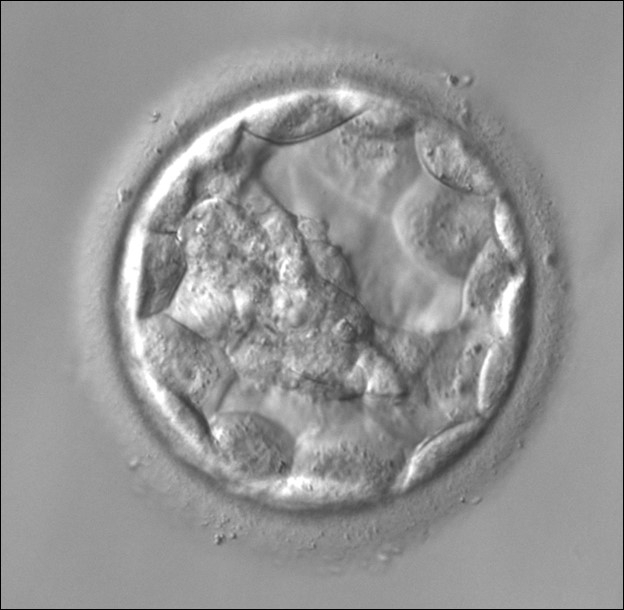
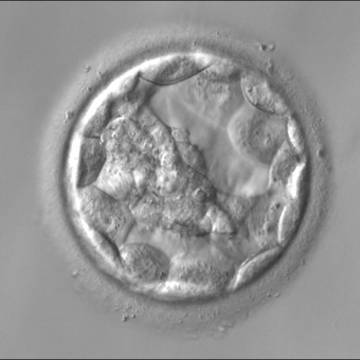
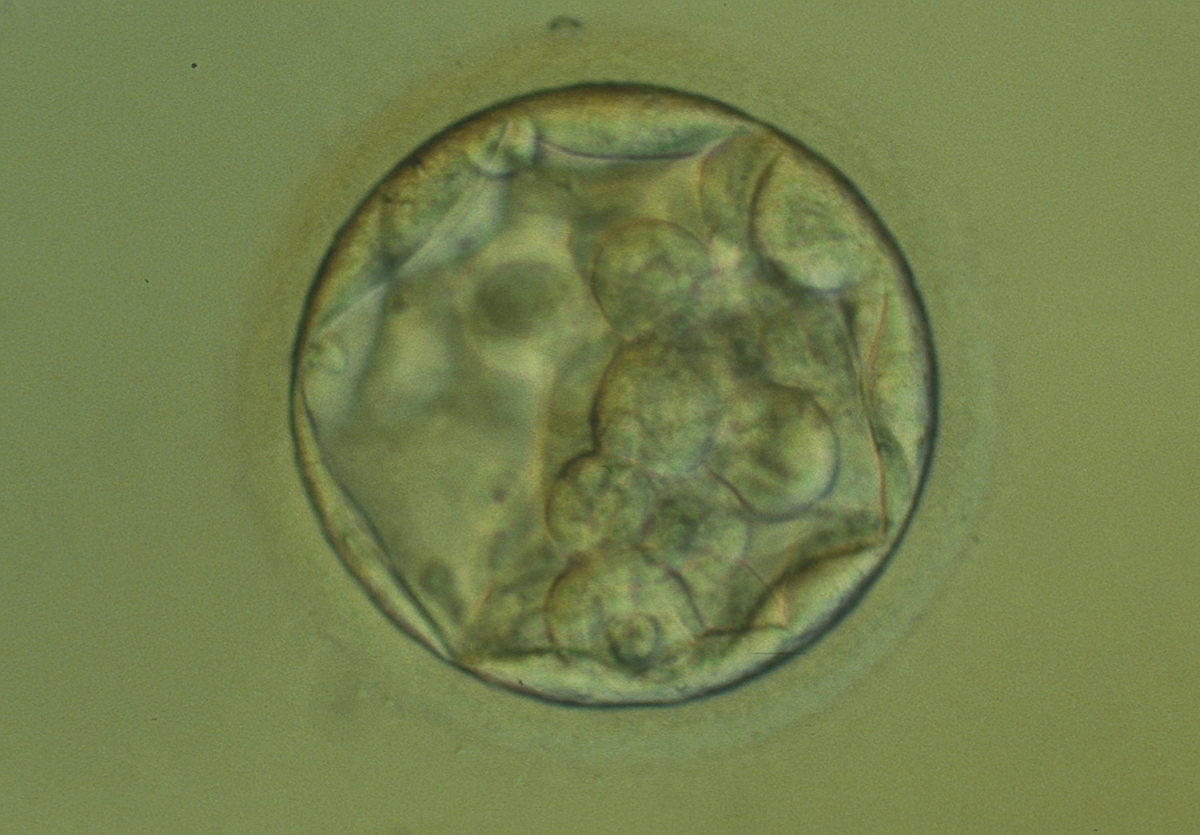
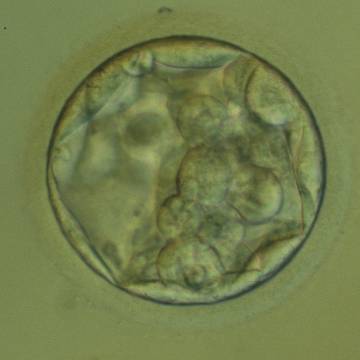
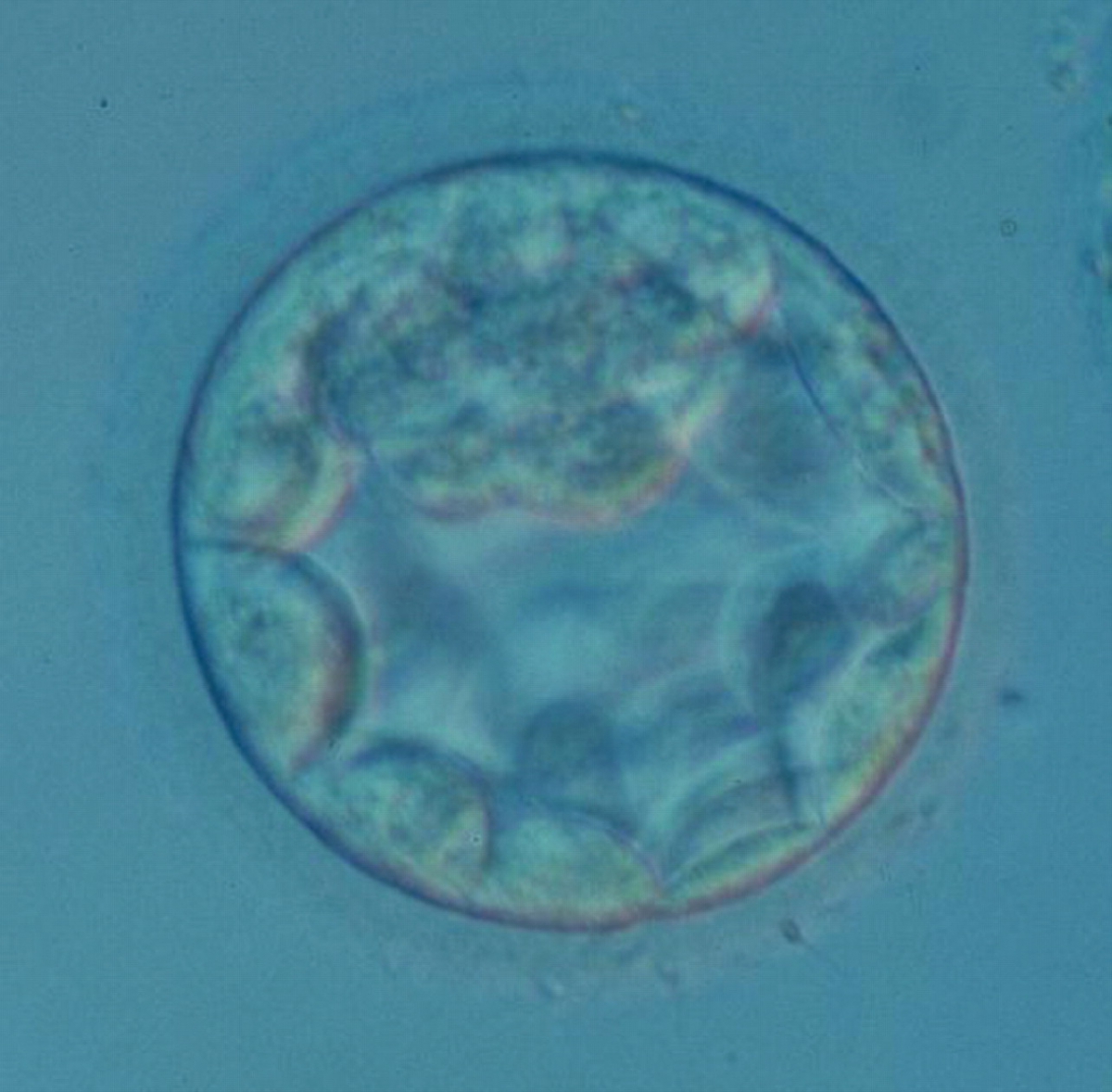
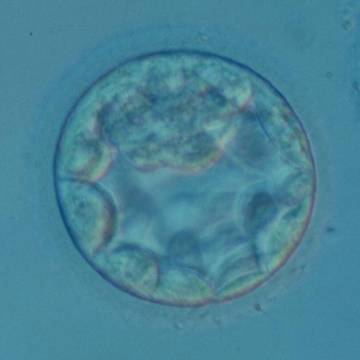
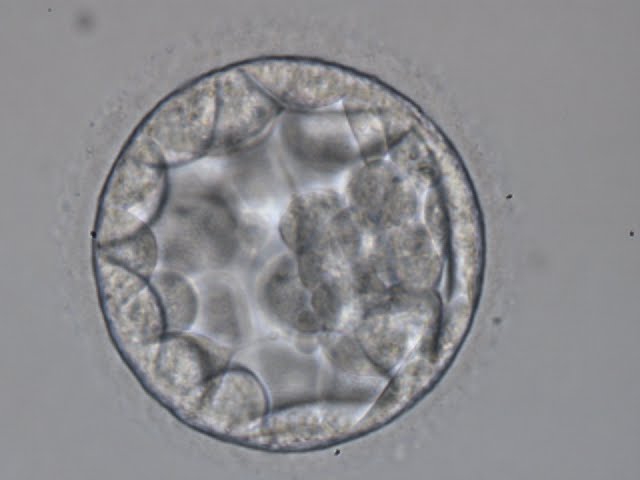
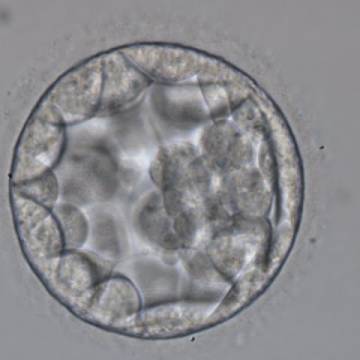
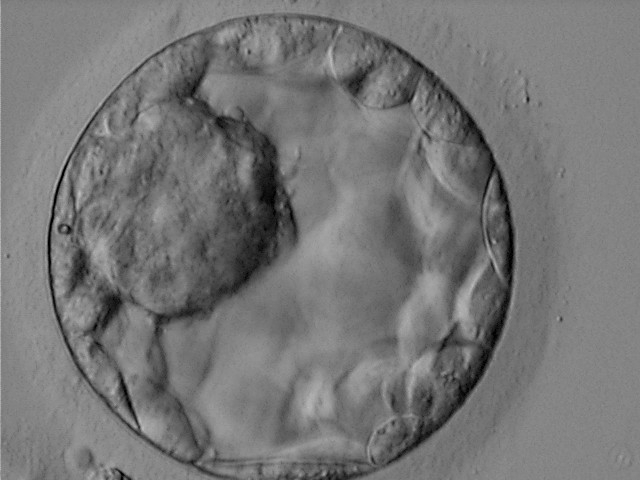
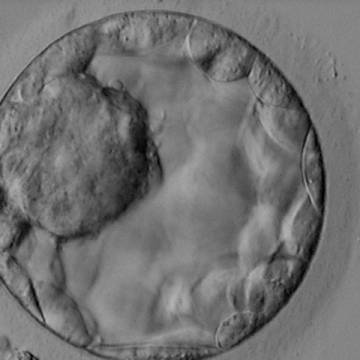
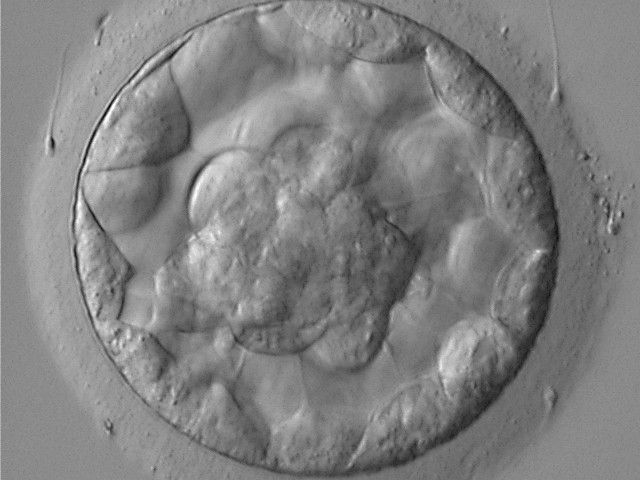
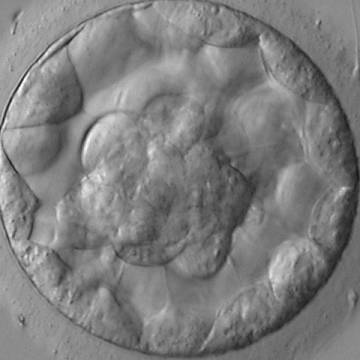
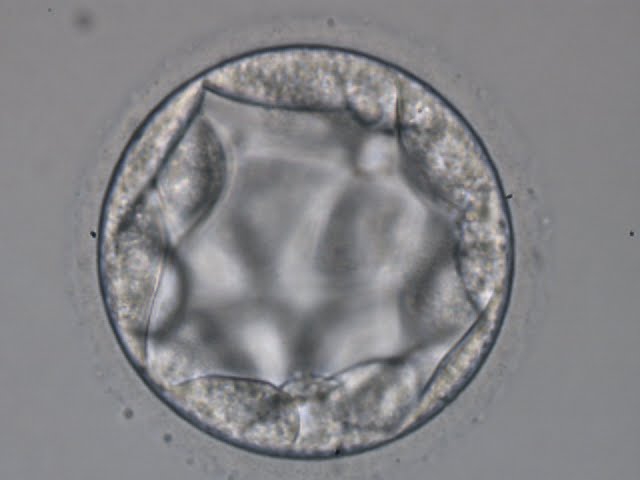
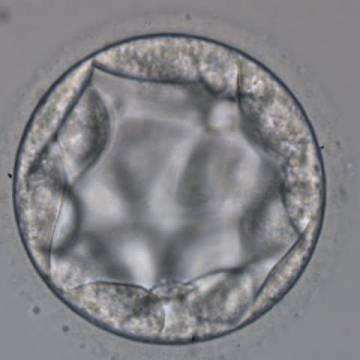
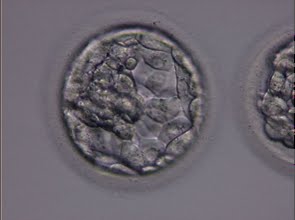
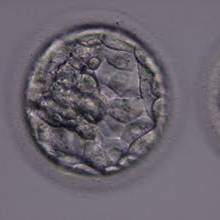
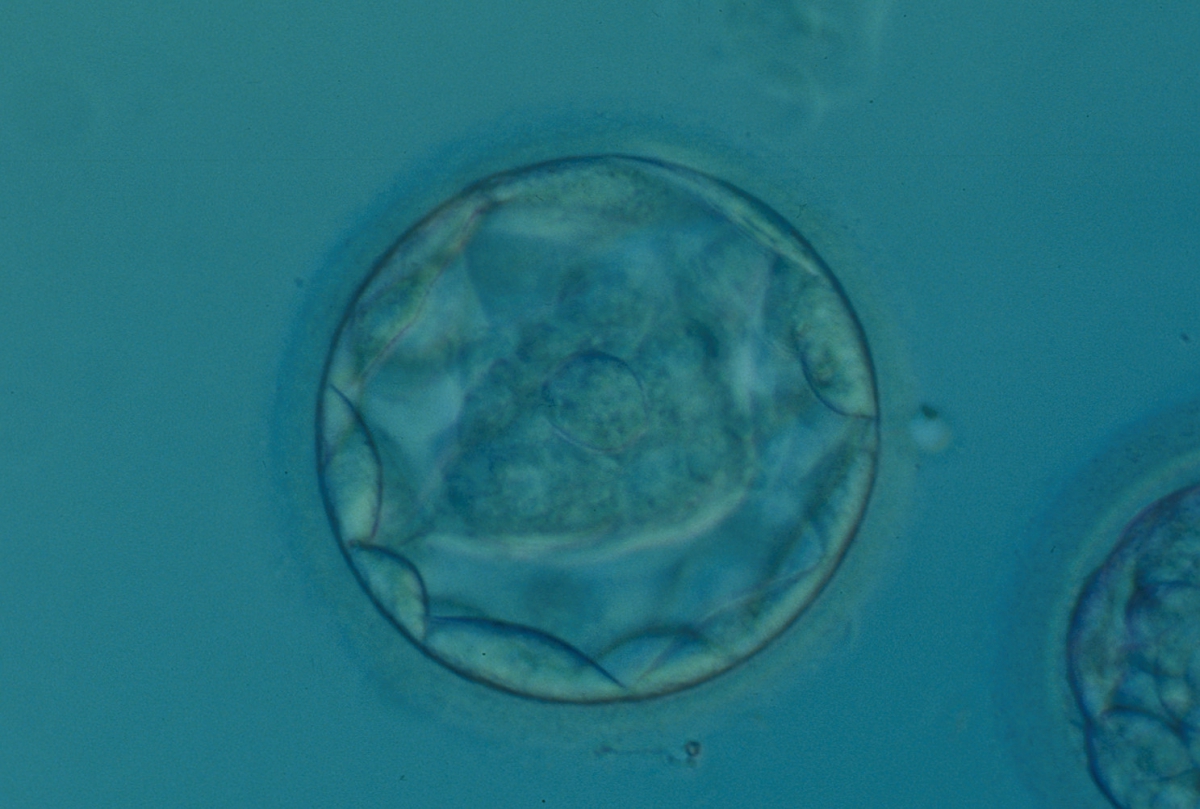
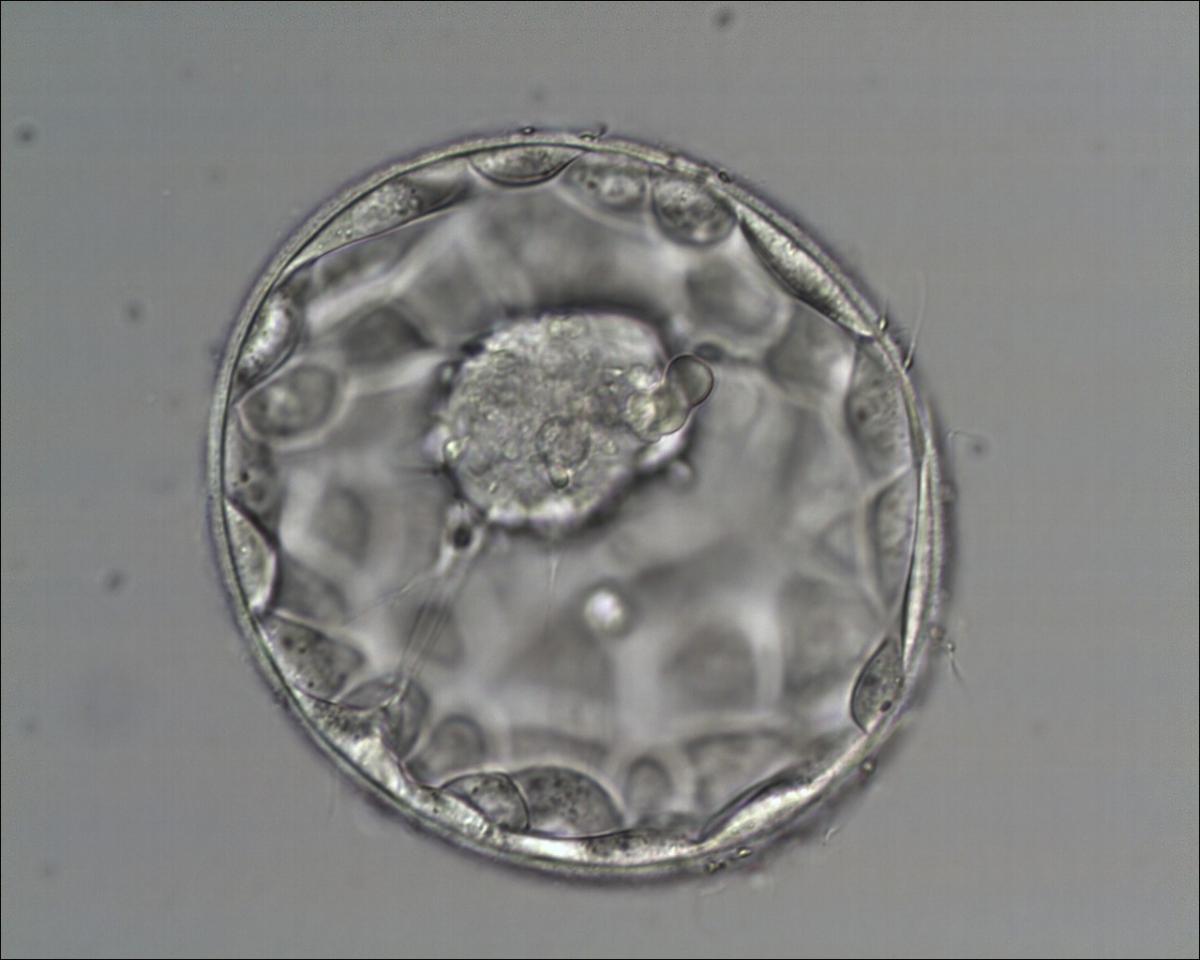
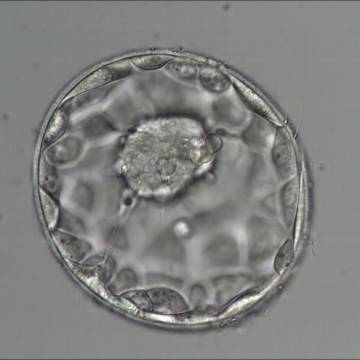
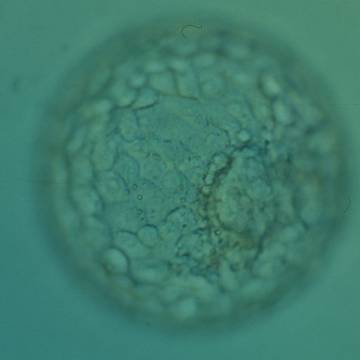
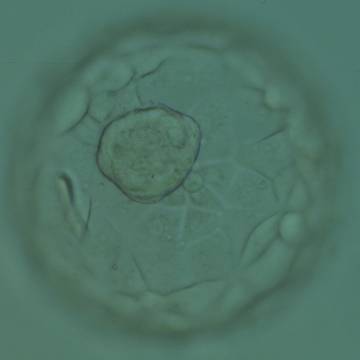
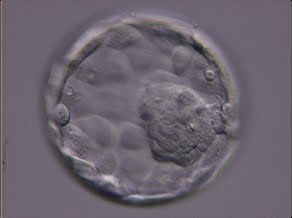
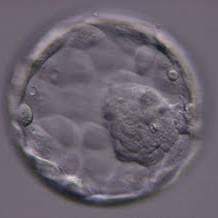
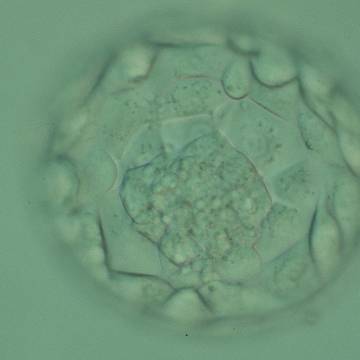
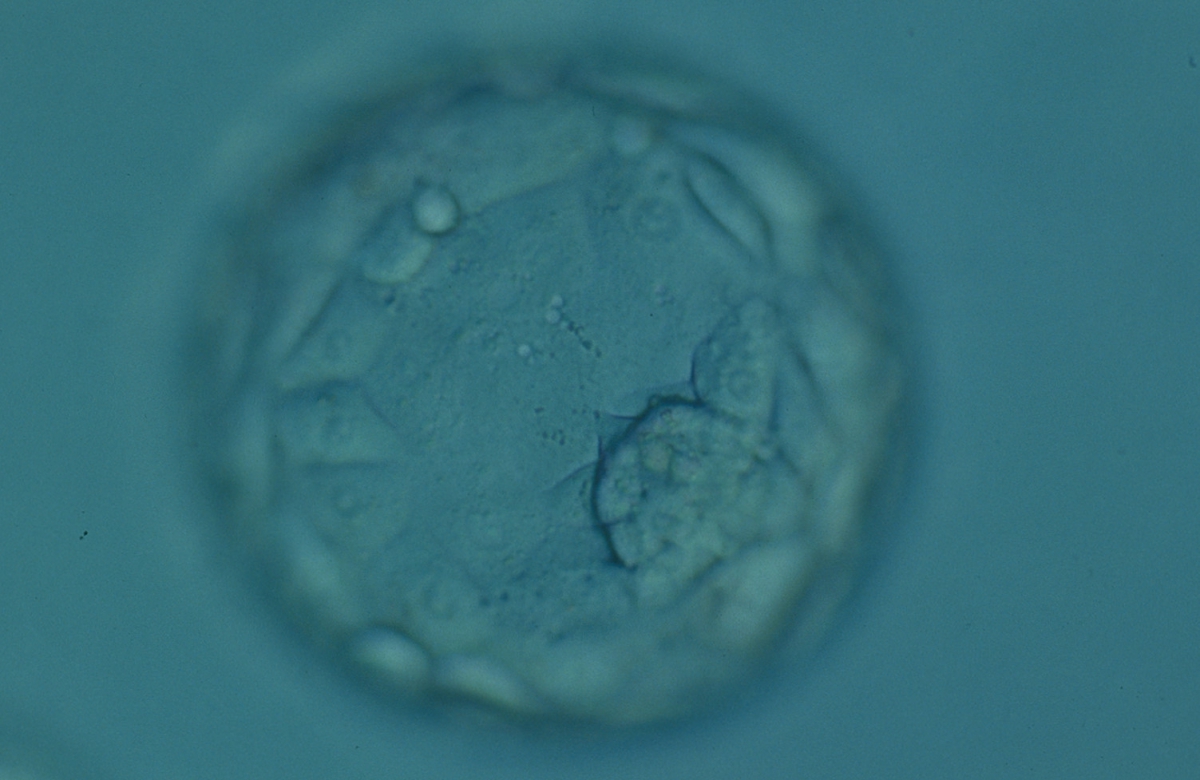
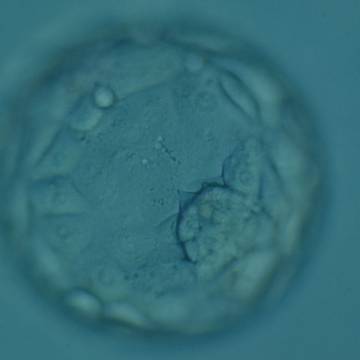
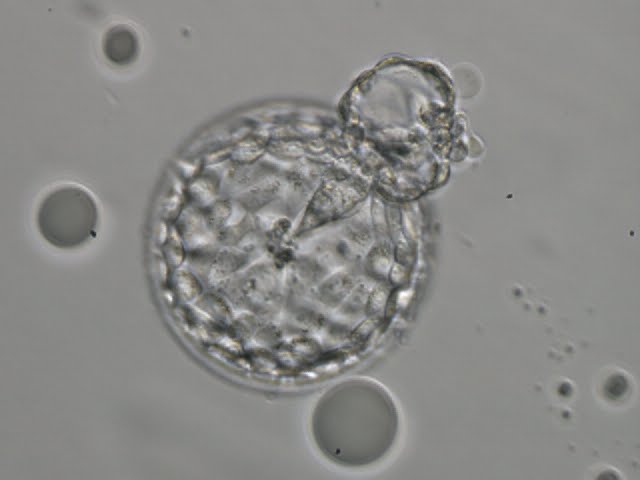
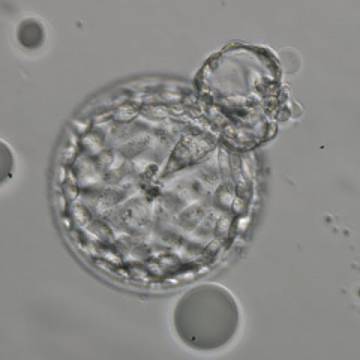
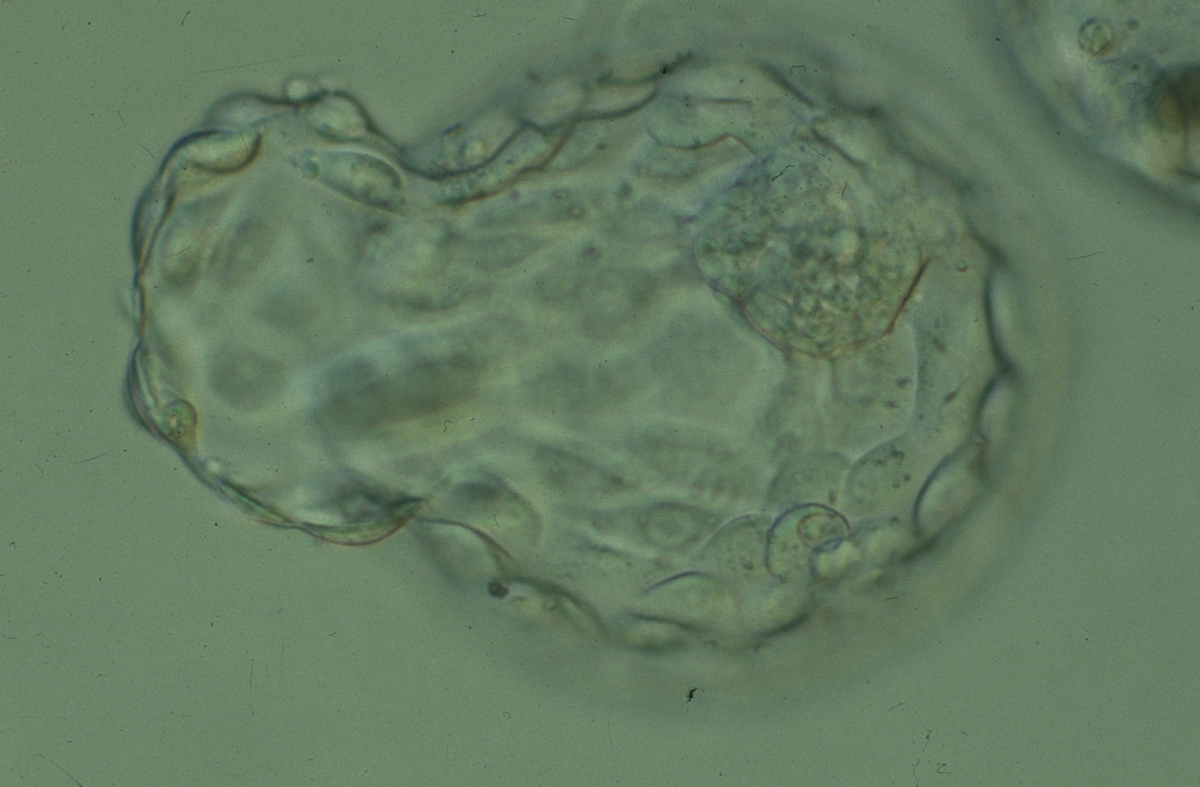
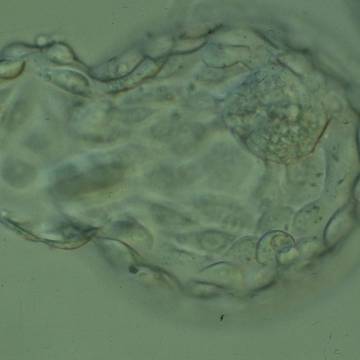
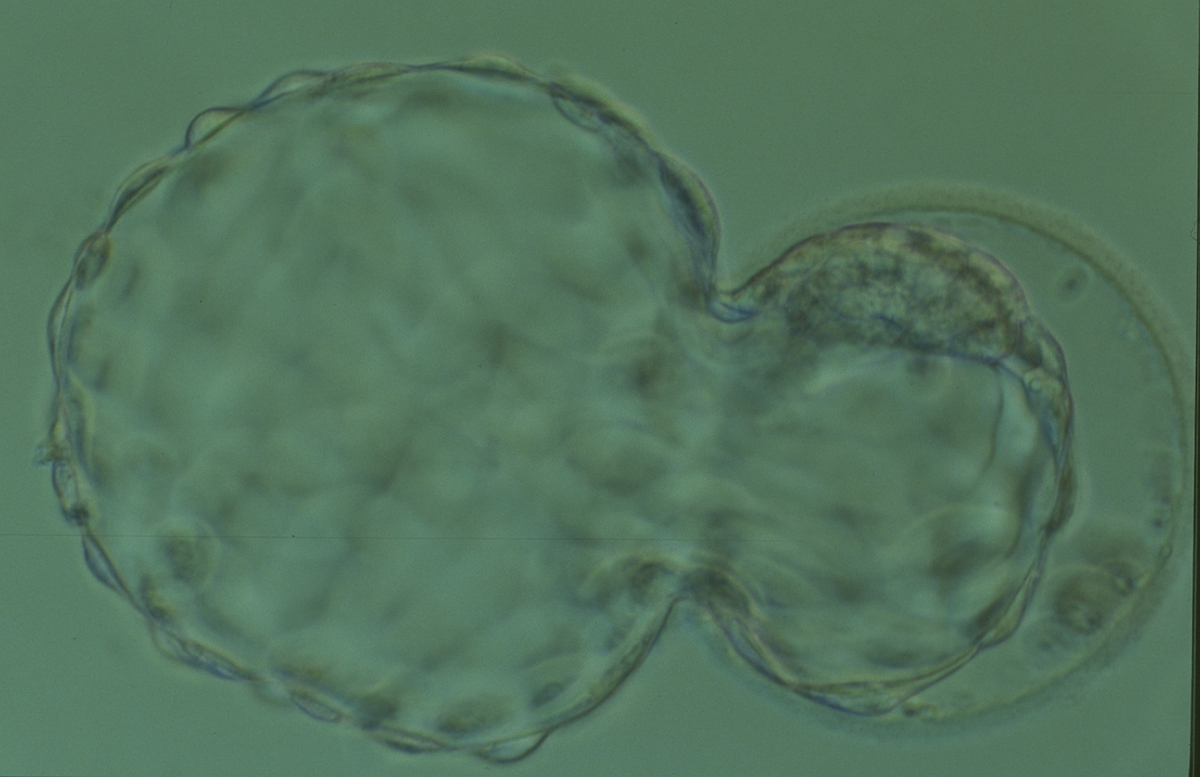
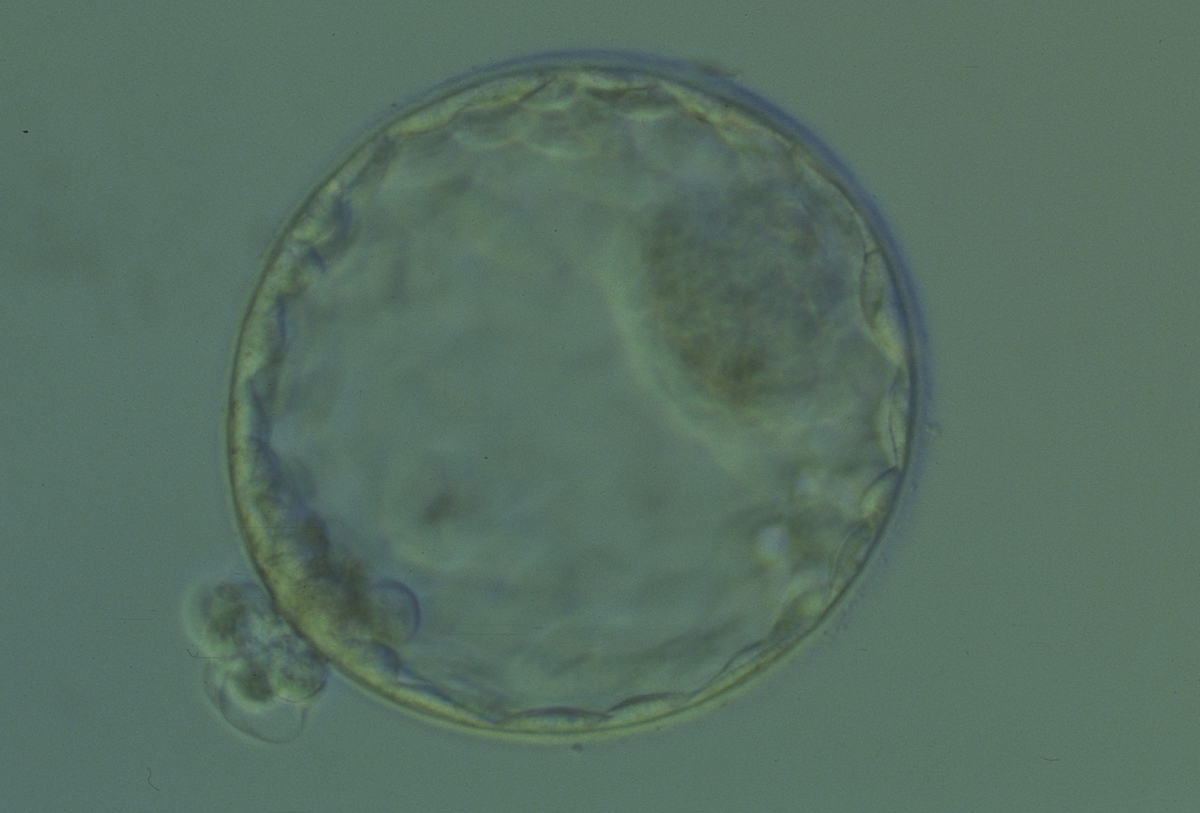
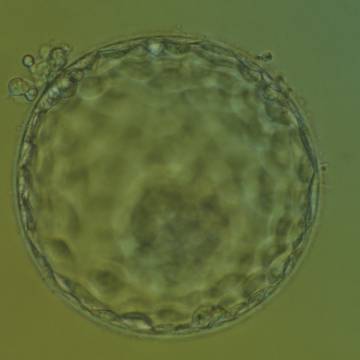
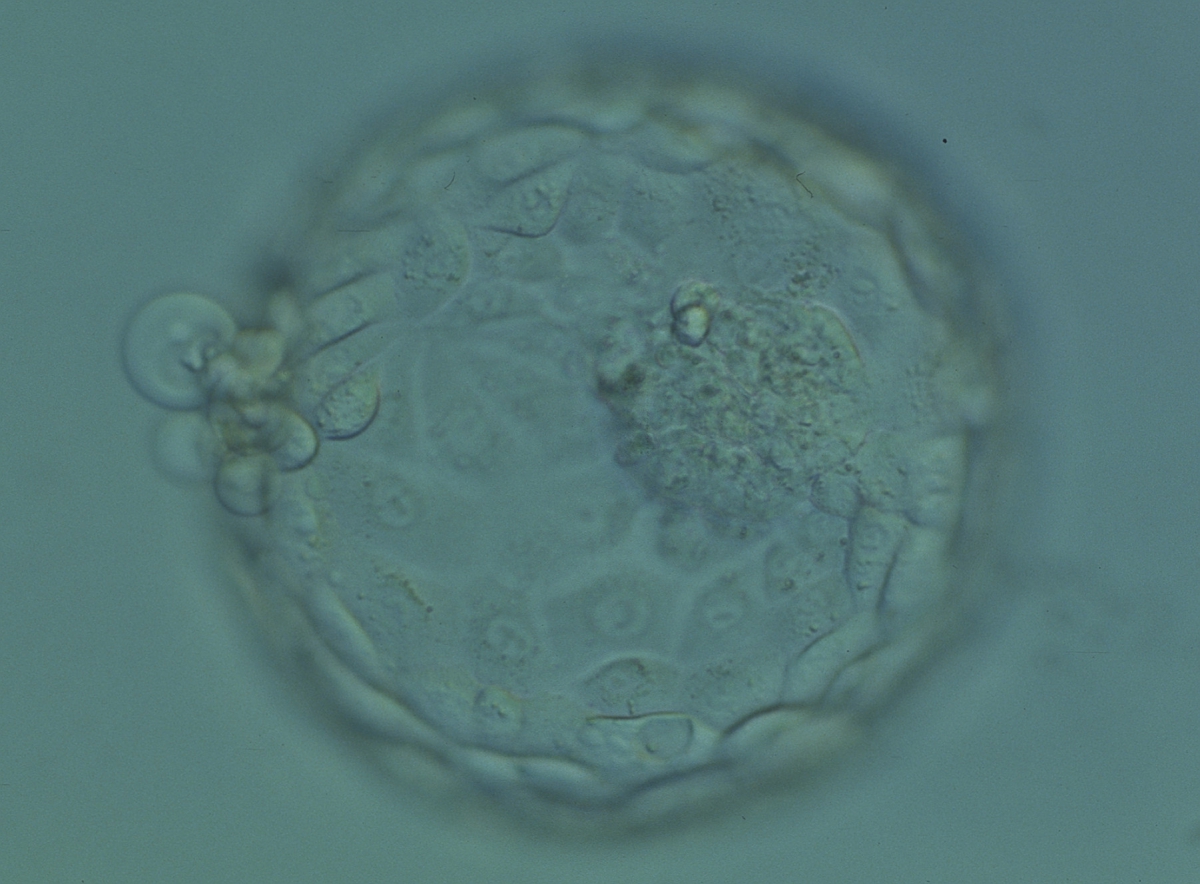
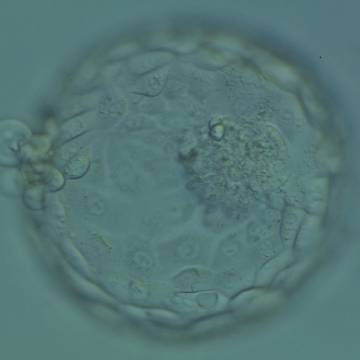
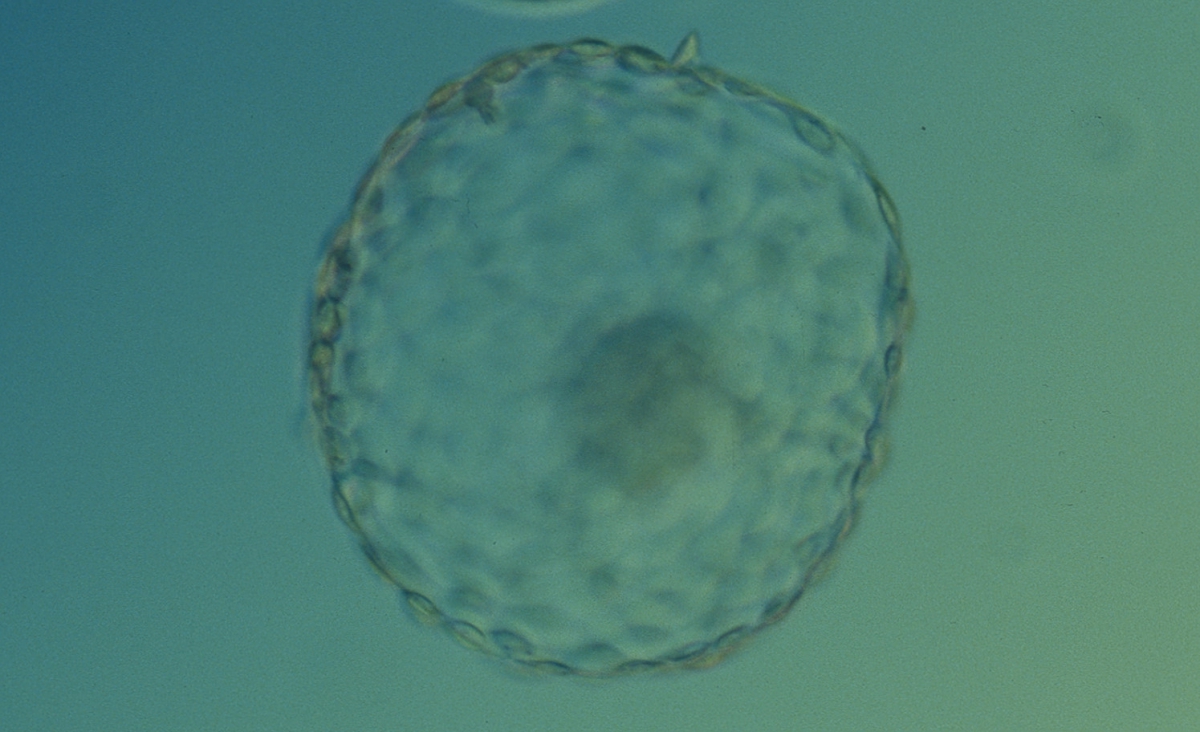
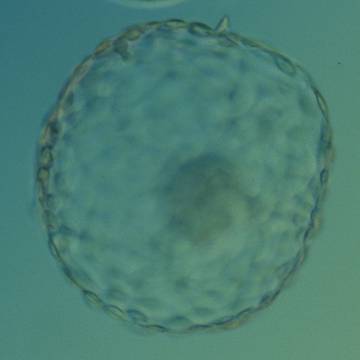
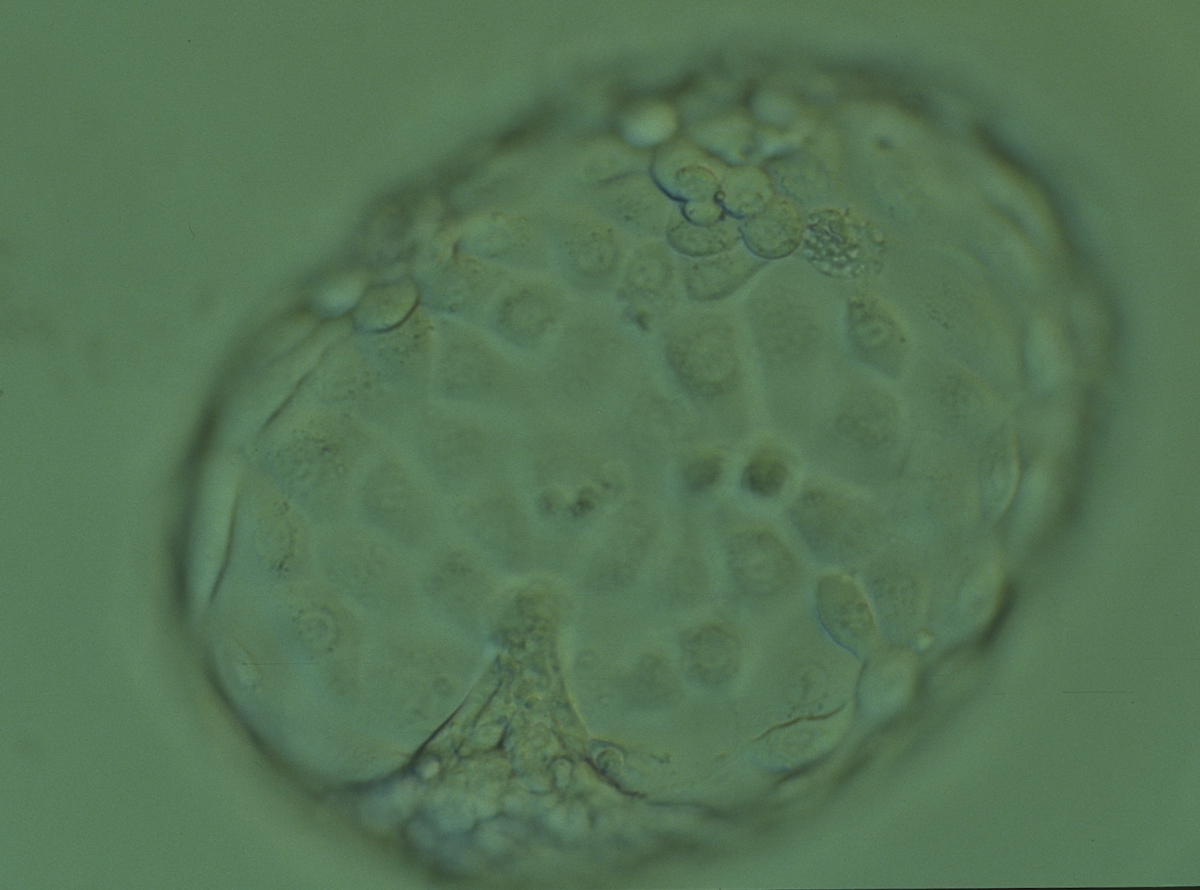
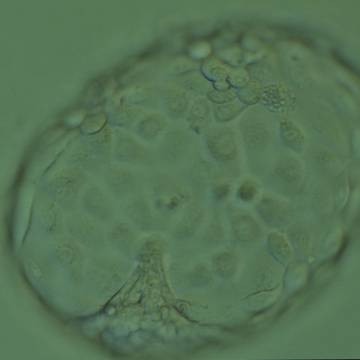
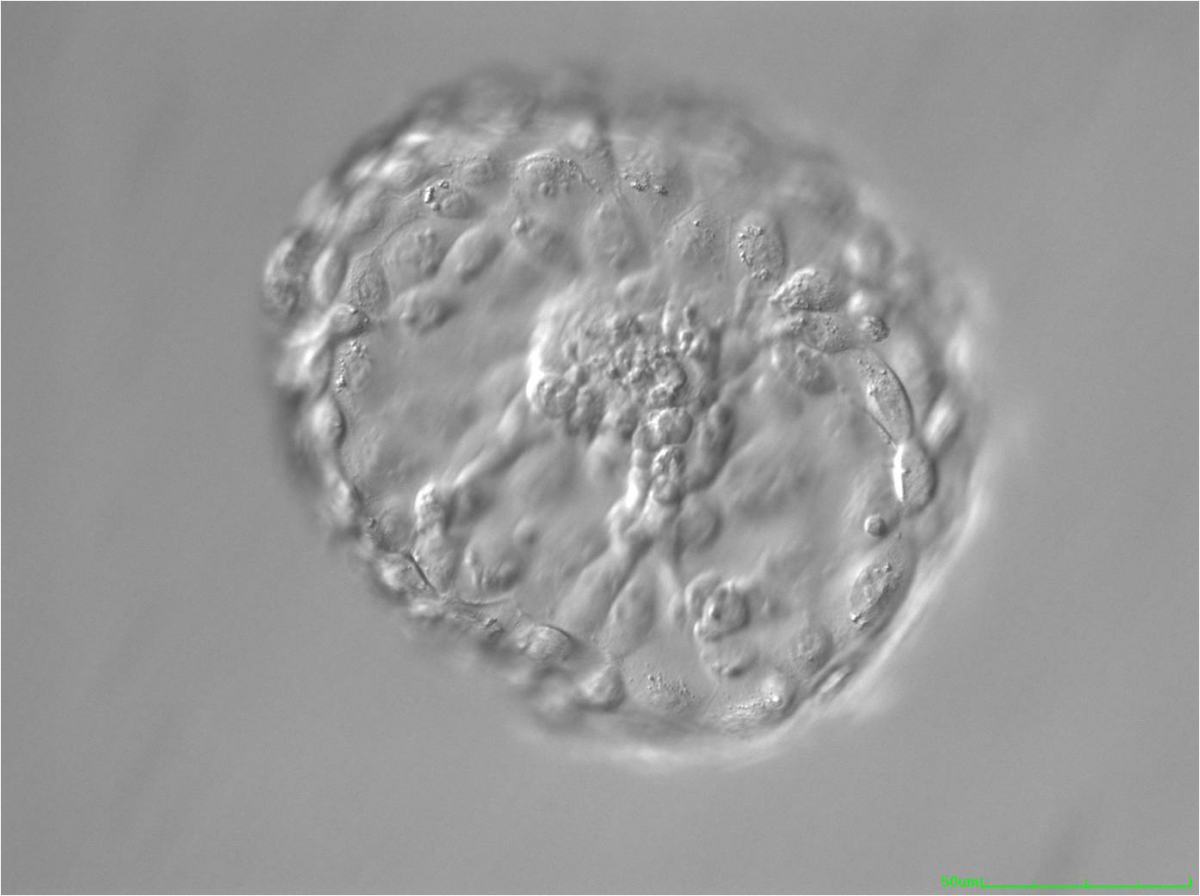
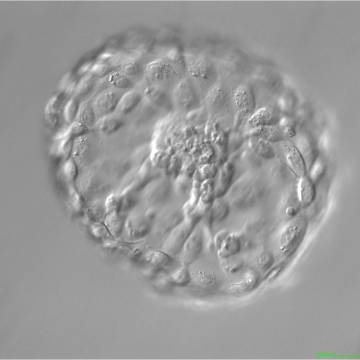
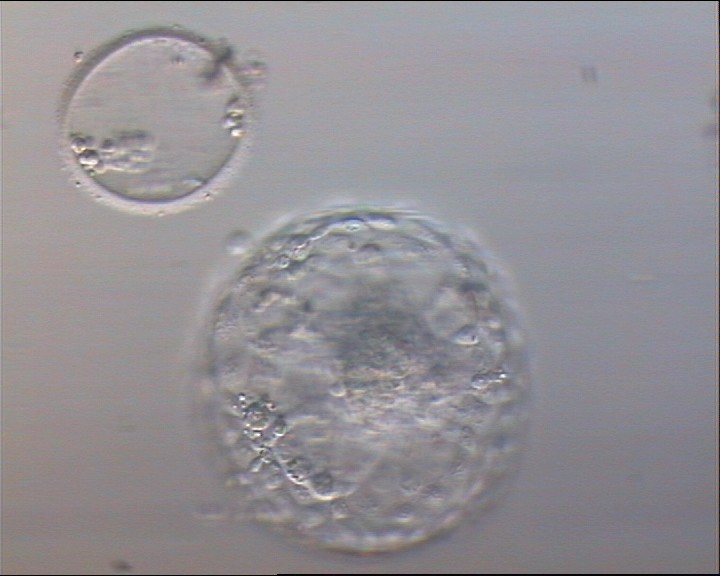
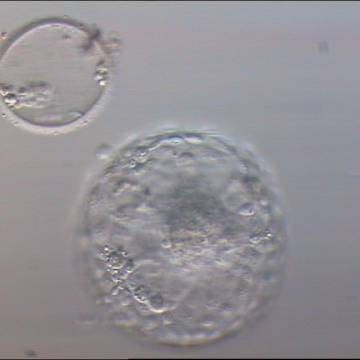
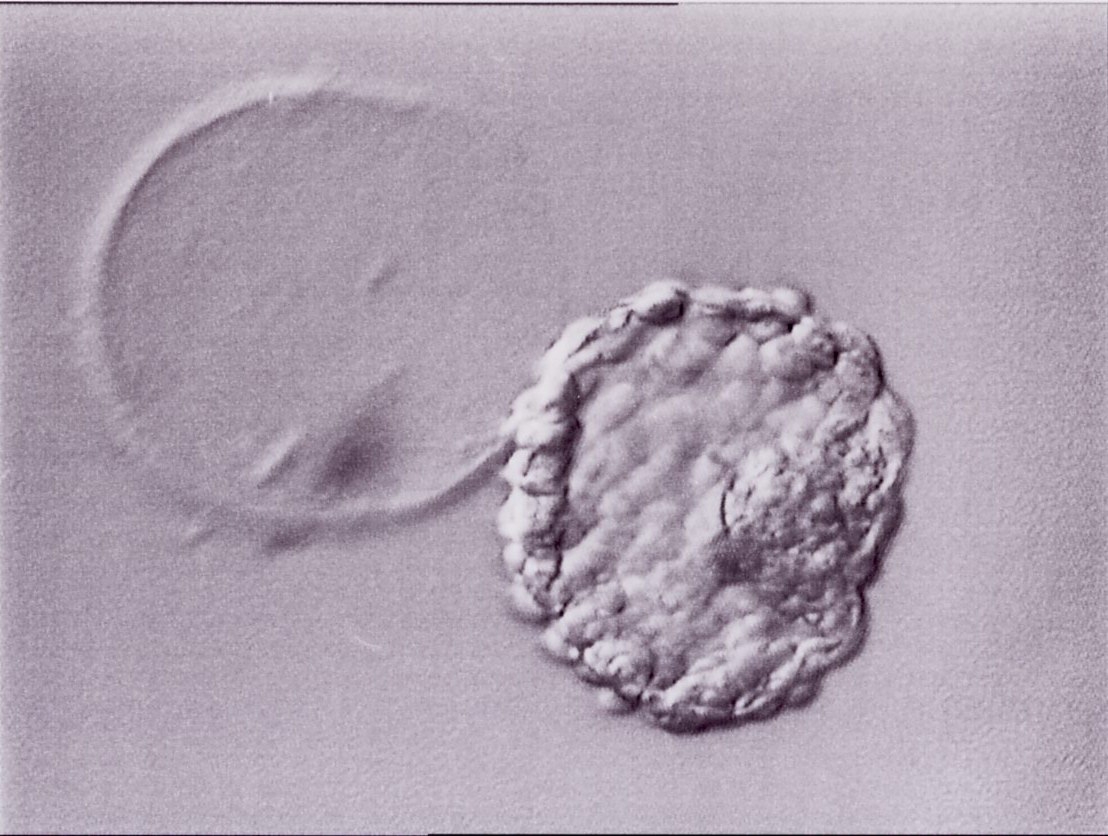
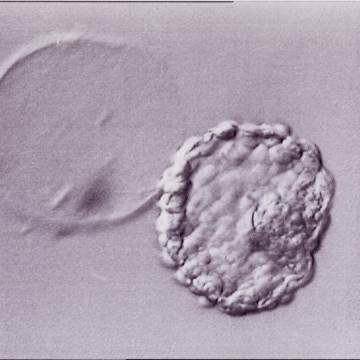
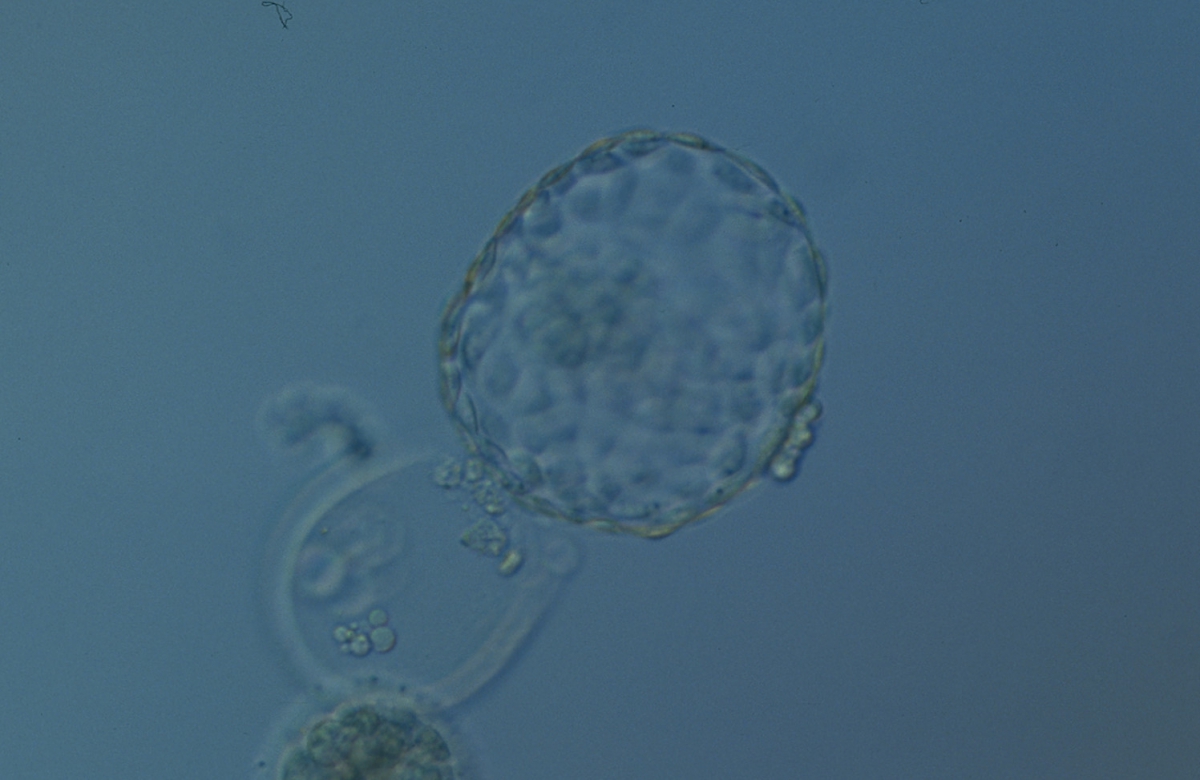
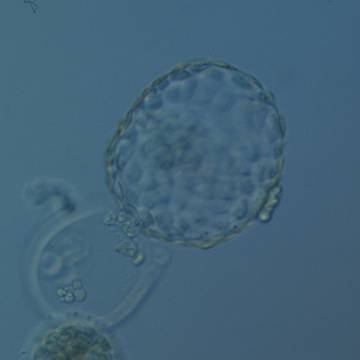
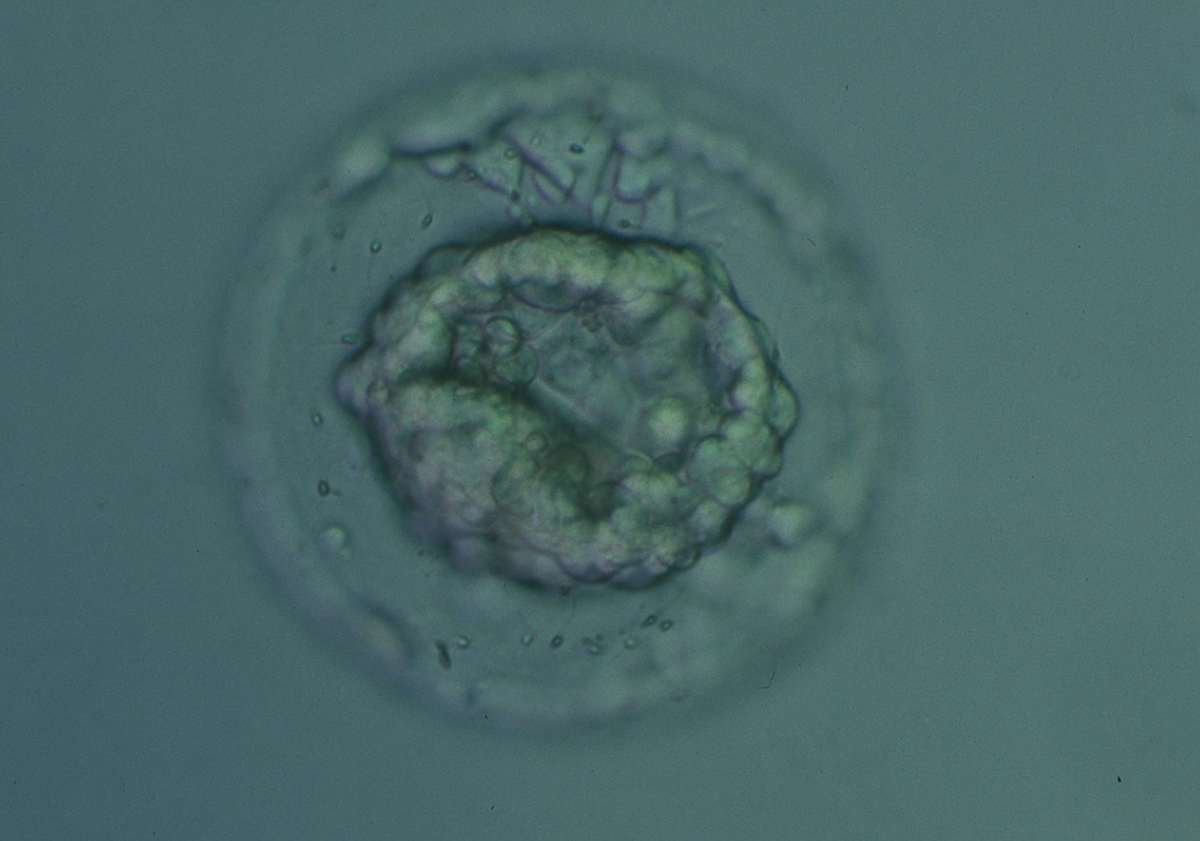
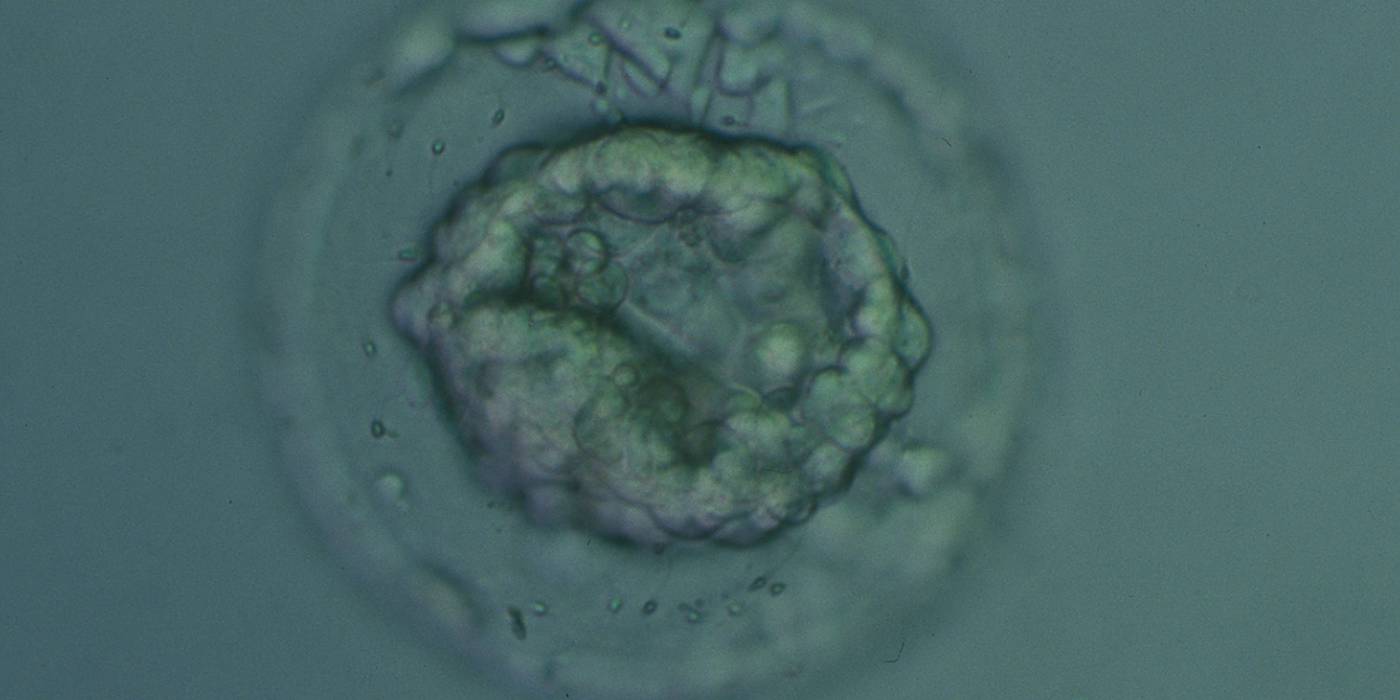
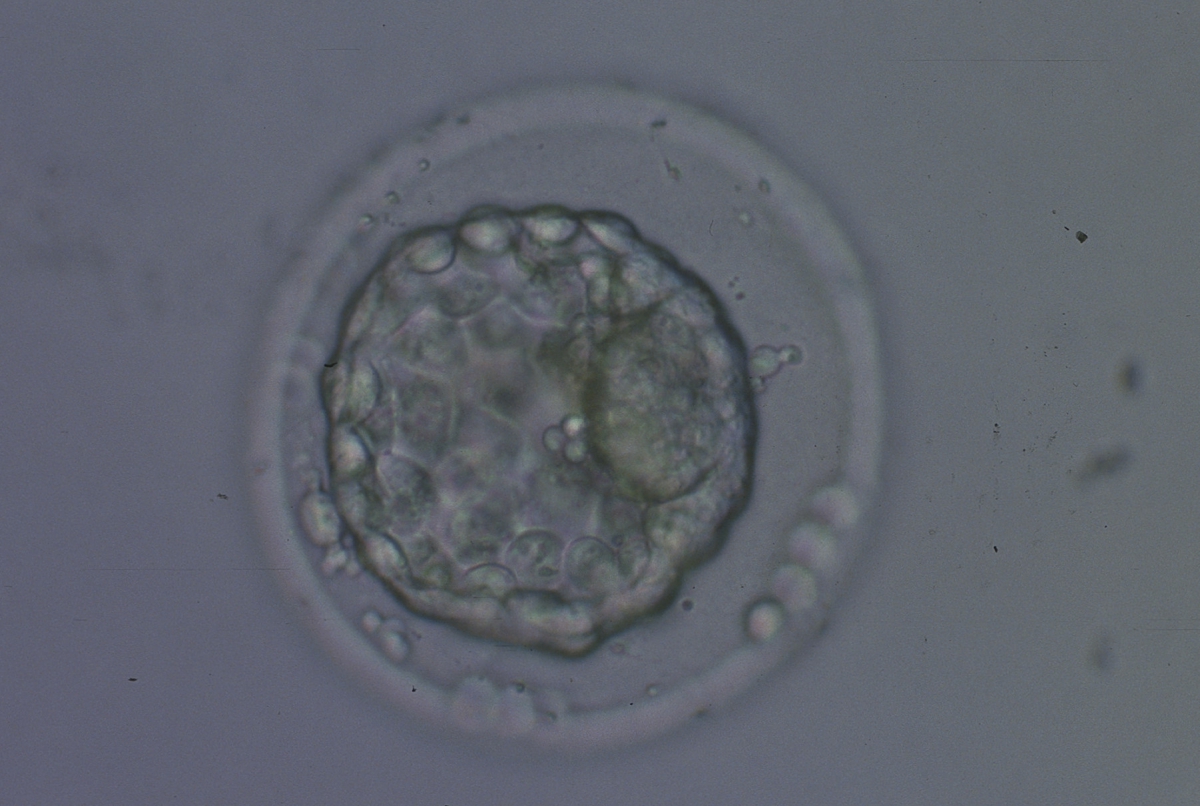
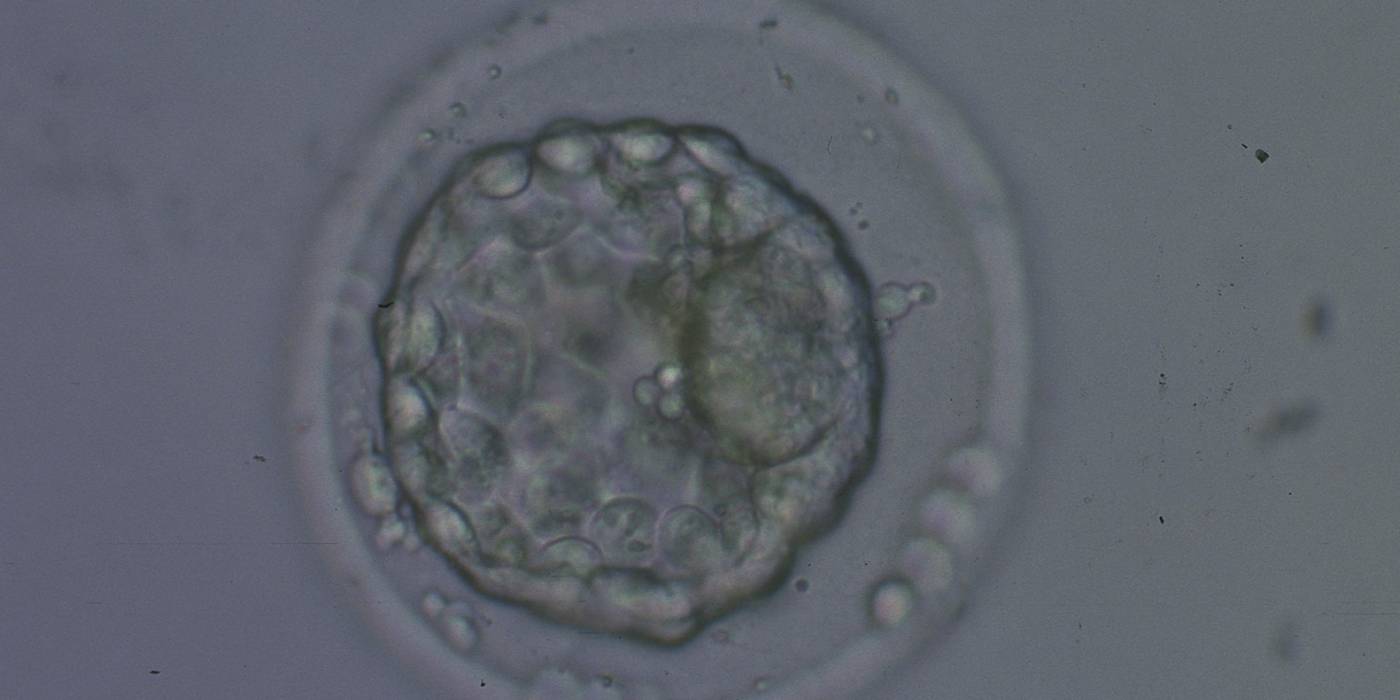
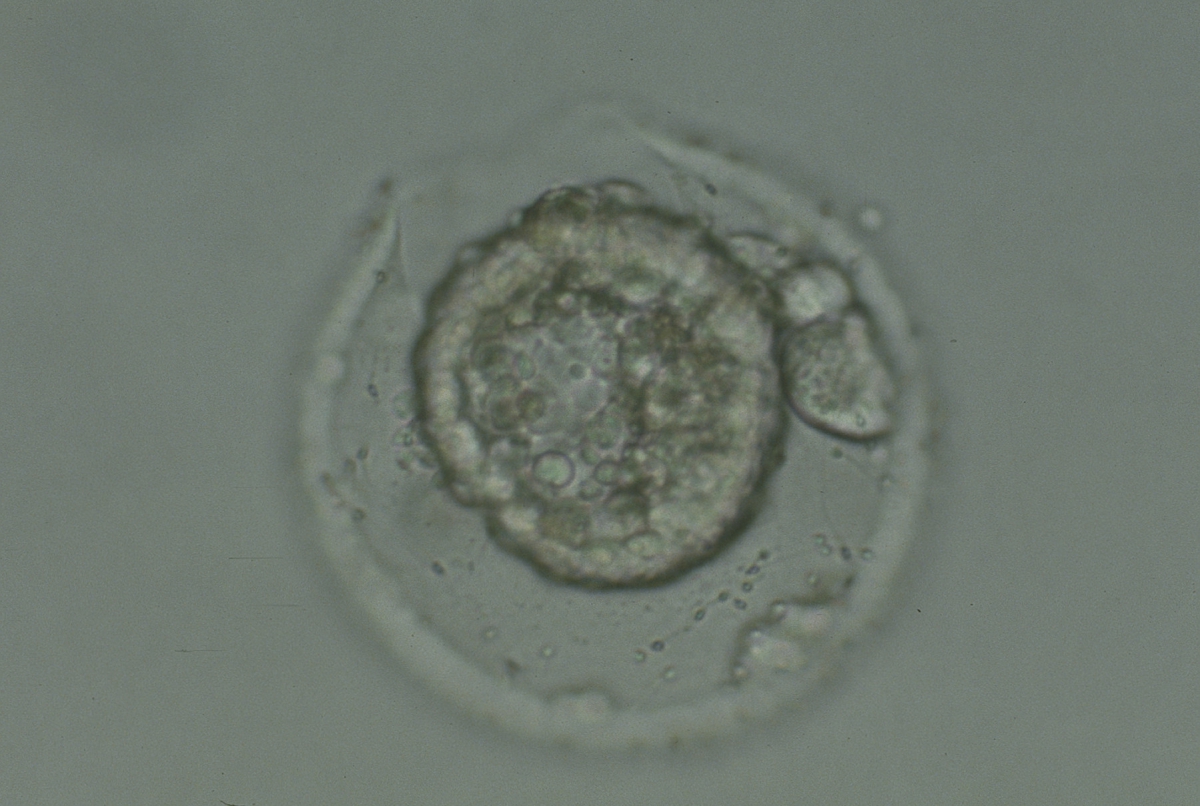
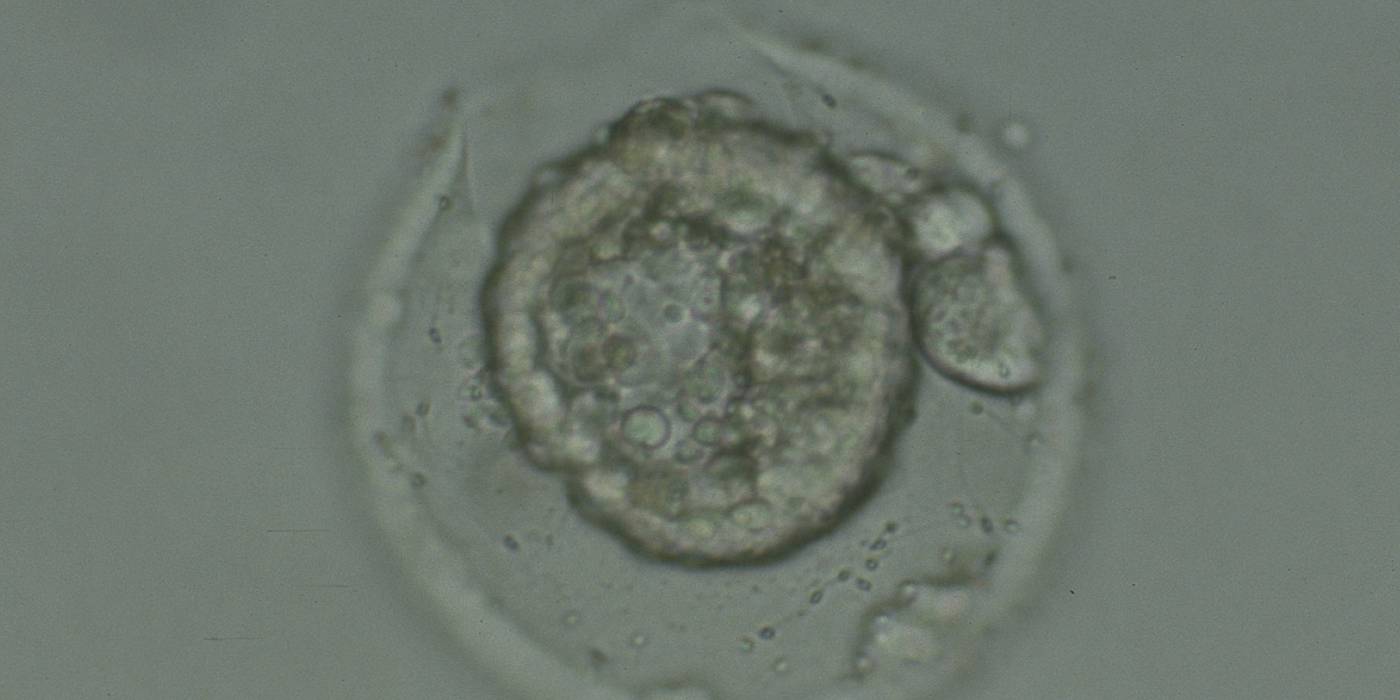
A. Degree of expansion
A defining moment in embryonic development is when fluid starts to accumulate between cells at the morulae stage of development. As the fluid's volume increases, a cavity appears gradually forming the blastocoel. This normally happens between Days 4 and 5 in human embryos in vitro and marks a new ‘era’ in the embryo's life, the blastocyst stage. As the fluid inside the newly formed blastocyst increases, so does the number of cells, and the combination of these two features causes a progressive enlargement of the blastocyst and it's cavity with a consequent progressive thinning of the zona pellucida (ZP). Finally, the blastocyst breaks free of the ZP through a process called hatching. The number of cells that comprise a blastocyst can vary considerably as shown in one study to range between 24 and 322 cells (Hardarson et al., 2003), which is often reflected in the blastocyst's morphology. The physiological events that underlie this transformation of a ‘cellular mass’ at the compaction stage to a highly structured blastocyst are not fully understood. However, cells that either by chance or fate are located in the outer part of the embryo start to flatten out, making contact with neighboring cells through tight junctions. In this way a barrier is created between the outside and the inside of the embryo, a prerequisite for blastocyst formation. Blastocyst formation is initiated through an initial secretion between the morula cells and this small cavity is then maintained and increased by actions of the membrane channels Na/K-ATPase that raise the salt concentration within the embryo, attracting water through osmosis (Watson et al., 2004). This increased water pressure gradually increases the size of the cavity which continues throughout the blastocyst stages.
The Istanbul consensus document (Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology, 2011) uses a simplified system of only four groups combining the first two and the last two groups of the Gardner and Schoolcraft (1999) grading system into two single groups which may be a limitation for the possibility to assess blastocysts. In this Atlas we have adopted the grading system that divides the grade of expansion into six categories and the ICM (Section B) and TE cell (Section C) grading into three categories similar to Gardner and Schoolcraft (1999) but have used the numerical scoring system suggested by the Istanbul consensus document: Grade 1 blastocysts are those in which the blastocoel cavity is less than half of the volume of the embryo (Figs 303–308); Grade 2 blastocysts are those in which the blastocoel cavity is half, or more than half, of the volume of the embryo (Figs 309–314); Grade 3 blastocysts are those in which the blastocoel cavity completely fills the embryo (Figs 315–320); Grade 4 blastocysts in which the blastocyst cavity is now greater than the original volume of the embryo and the ZP is thinned (Figs 321–326); Grade 5 blastocysts or hatching blastocysts in which the blastocoel cavity is greater than the original volume of the embryo and the TE is herniating through a natural breach in the ZP (Figs 327–332) and Grade 6 blastocyts or hatched blastocysts are those in which the blastocyst has completely escaped from a natural breach in the ZP (Figs 333–338). The latter two grades should be distinguished from blastocysts that are hatching or have hatched from an artificial breach in the ZP created by an assisted hatching procedure or following embryo biopsy whereby the breach in the ZP is quite large, permitting the blastocyst to escape earlier and well before complete expansion of the blastocoel cavity. The artificially hatched blastocyst could therefore contain far fewer cells than those that undergo natural hatching.
It is not uncommon to observe blastocysts that have collapsed or are in the process of collapsing (Figs 339–341). In this instance it is difficult to accurately grade the blastocysts and the consensus document (Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology, 2011) suggests that 1–2h is allowed to elapse before the blastocyst is re-assessed as regular cycles of expansion and collapse are normal and can be observed even without intervention as has been recorded using continuous time-lapse recording within the incubator.
The timing and grade of blastocyst expansion has been shown by several investigators to be an important predictor of implantation (Dokras et al., 1993; Gardner et al., 2000; Shapiro et al., 2008; Ahlström et al., 2011). It must, however, be remembered that embryologists have traditionally chosen to transfer the most developed blastocyst with the highest grade of expansion when available for transfer. No randomized, controlled trial comparing transfer of good quality blastocysts of a lower grade when they are present within a cohort that includes blastocysts with higher grades has been undertaken. Furthermore, recent evidence suggests that human female embryos undergo X chromosome inactivation from the 8-cell cleavage stage to the blastocyst stage of development which takes some time, therefore meaning that female blastocysts may be less expanded than their male sibling blastocysts but may be just as viable (van den Berg et al., 2009).
Article references:
Ahlström A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting pregnancy and birth after single blastocyst transfer. Hum Reprod 2011;26:3289-3296.
Abstract/FREE Full Text
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 2011;26:1270-1283.
Abstract/FREE Full Text
Dokras A, Sargent IL, Barlow DH. Human blastocyst grading: an indicator of developmental potential? Hum Reprod 1993;8:2119-2127.
Abstract/FREE Full Text
Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Toward Reproductive Certainty: Fertility and Genetics Beyond 1999. UK: Parthenon Publishing London; 1999. p. 378-388.
Google Scholar
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 2000;73:1155-1158.
CrossRef | Medline | Web of Science | Google Scholar
Hardarson T, Caisander C, Sjögren A, Hanson C, Hamberger L, Lundin K. A morphological and chromosomal study of blastocysts developing from morphologically suboptimal human preembryos compared to control blastocysts. Hum Reprod 2003;18:399-407.
Abstract/FREE Full Text
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Thomas S. Large blastocyst diameter, early blastulation, and low preovulatory serum progesterone are dominant predictors of clinical pregnancy in fresh autologous cycles. Fertil Steril 2008;90:302-309.
CrossRef | Medline | Web of Science | Google Scholar
Van den Berg IM, Laven JS, Stevens M, Jonkers I, Galjaard RJ, Gribnau J, van Doorninck JH. X chromosome inactivation is initiated in human preimplantation embryos. Am J Hum Genet 2009;84:771-779.
CrossRef | Medline | Web of Science | Google Scholar
Watson AJ, Natale DR, Barcroft LC. Molecular regulation of blastocyst formation. Anim Reprod Sci 2004;82–83:583-592.
Google Scholar