D. Nucleation
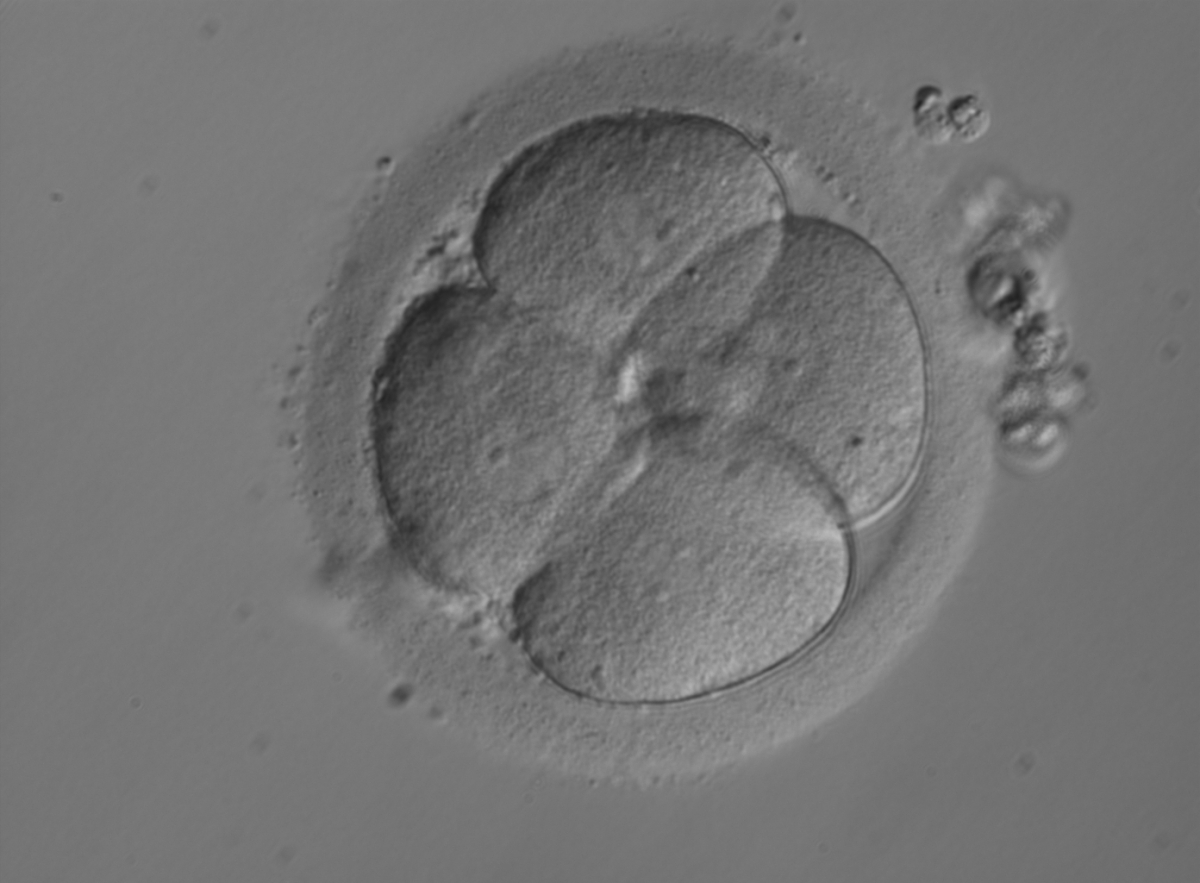
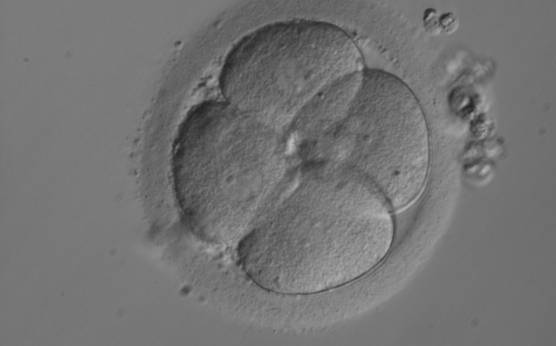
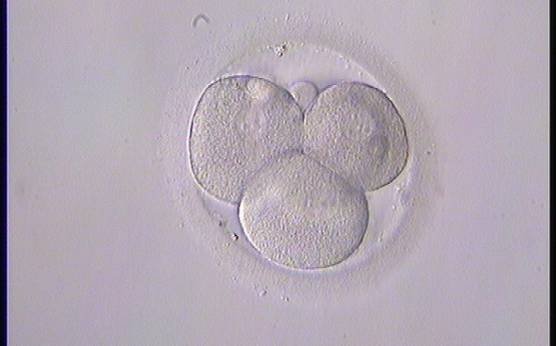
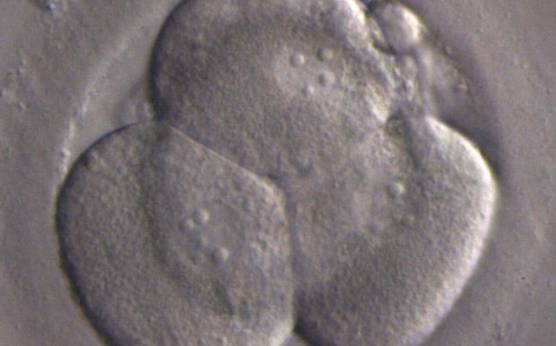
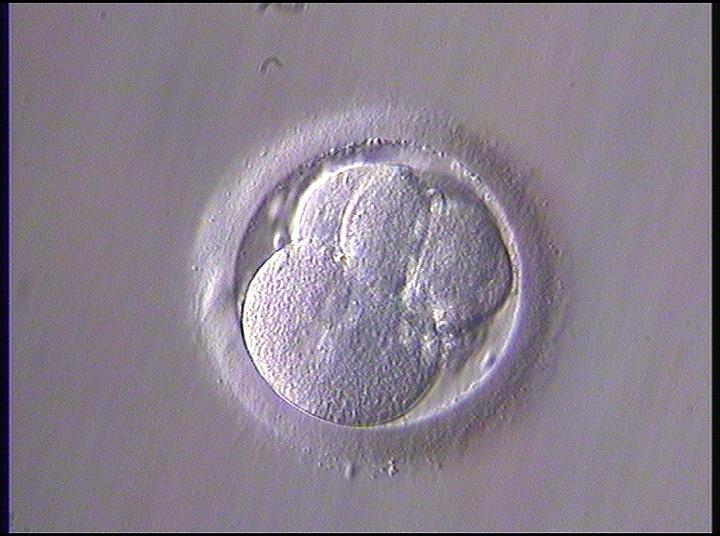
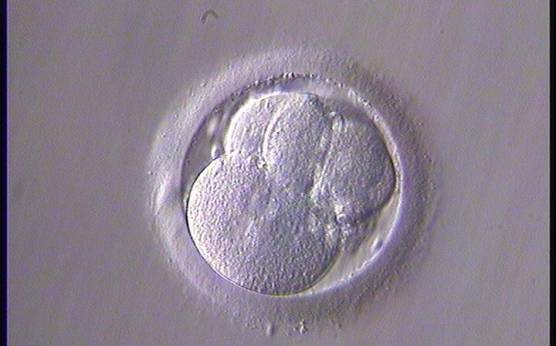
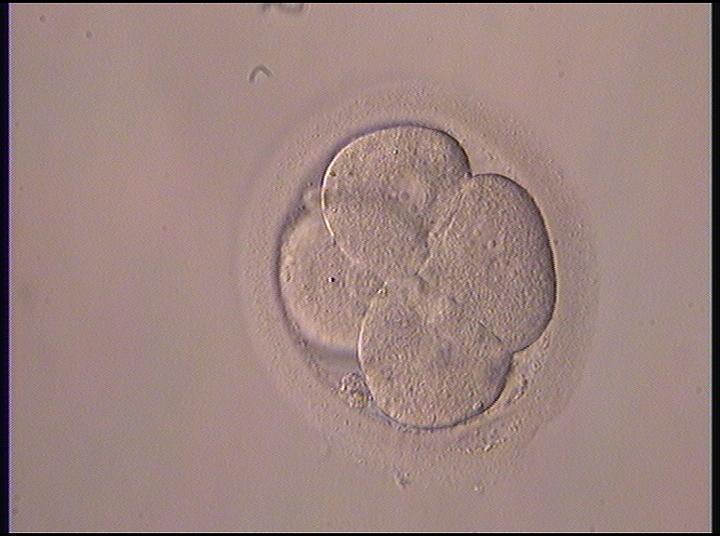
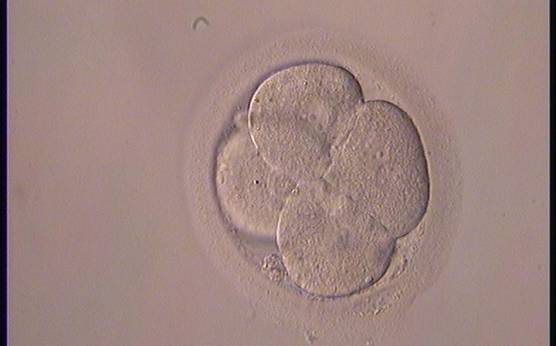
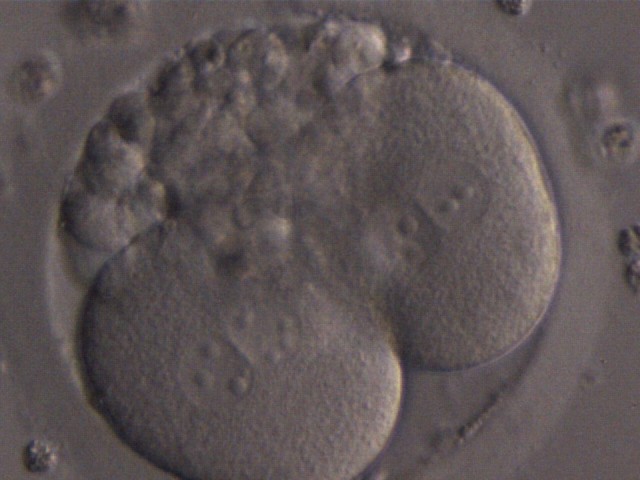
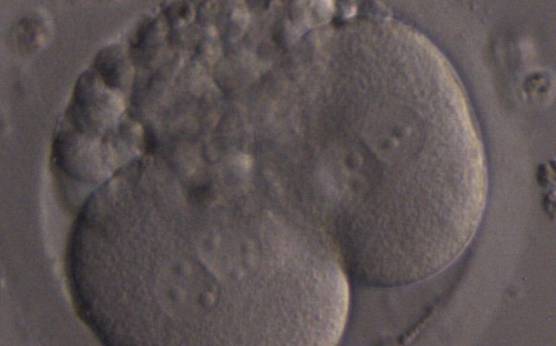
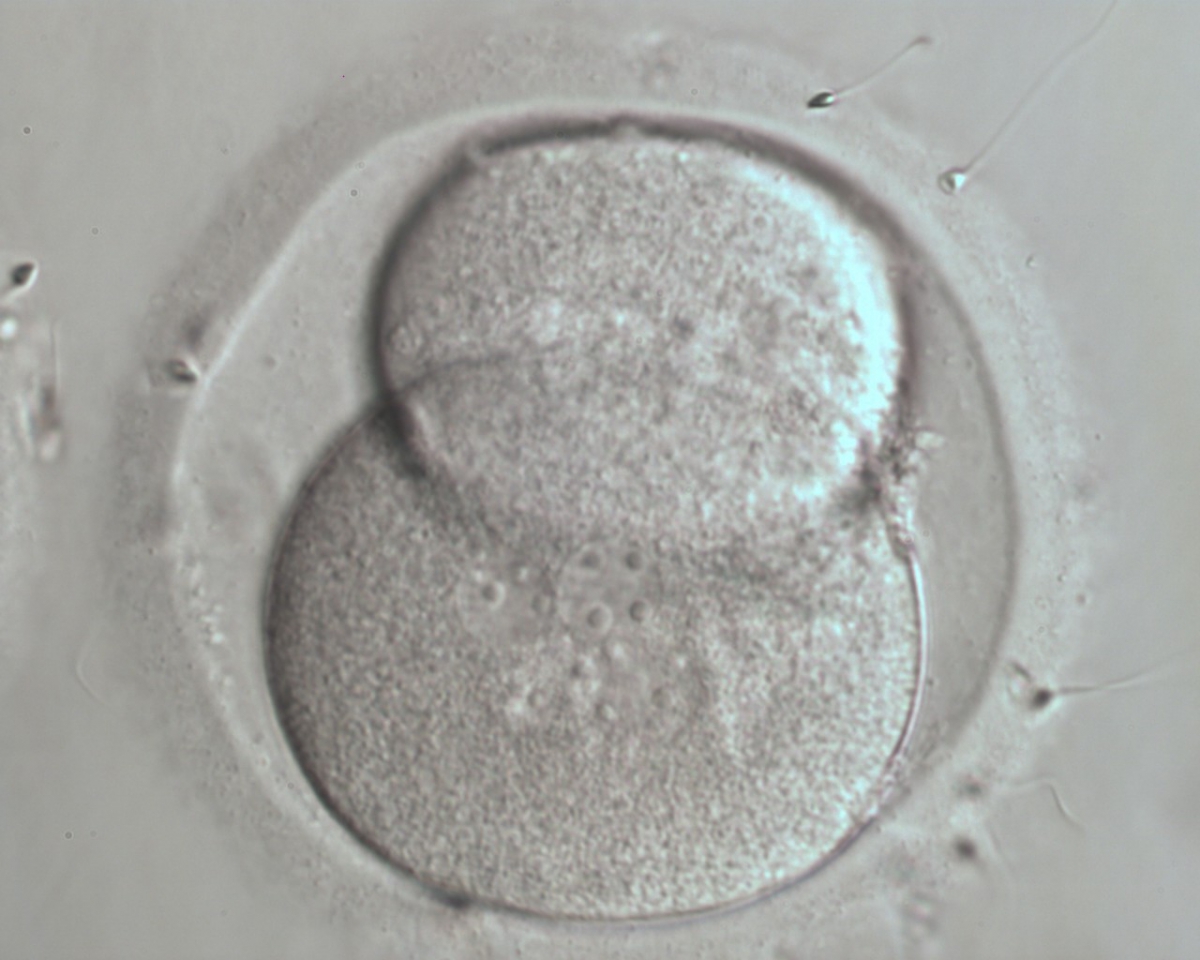
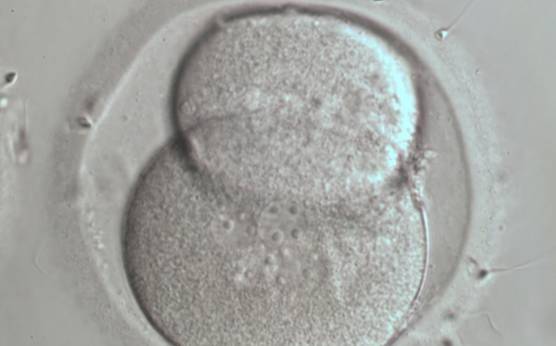
The nucleation status is defined as the presence or absence of nuclei in the blastomeres of the cleavage stage embryo. Ideally, the nucleation status of each blastomere in the embryo should be evaluated as a single nucleus per blastomere (Figs 264–266), no nuclei visible or multinucleation (Figs 267–270).
The most studied nucleation status is multinucleation, which is defined as the presence of more than one nucleus in at least one blastomere of the embryo (Jackson et al., 1998; Van Royen et al., 2003). Multinucleation can be evaluated both in the early cleaved Day 1 (26–28±1 h post-insemination), Day 2 (44 ± 1 h post-insemination) and Day 3 (68 ± 1 h post-insemination) cleavage stage embryos, although the assessment of a Day 3 embryo may be more complicated due to the smaller cell size and the larger number of cells (Van Royen et al., 2003). Embryo quality has been shown to correlate with multinucleation, and 4-cell embryos on Day 2 and 8-cell embryos on Day 3 show reduced multinucleation compared with the other cell stages observed on these days (Van Royen et al., 2003; Ziebe et al., 2003).
Multinucleation is predictive of a decreased implantation potential (Jackson et al., 1998; Pelinck et al., 1998; Van Royen et al., 2003; Moriwaki et al., 2004) and multinucleated embryos are associated with an increased level of chromosome abnormalities (Pickering et al., 1995; Hardarson et al., 2001; Agerholm et al., 2008) as well as an increased risk of spontaneous abortion (Scott et al., 2007). Multinucleation is more frequent in blastomeres originating from embryos with uneven cleavage compared with embryos with evenly cleaved blastomeres (Hardarson et al., 2001).
Multinucleation can also be divided into binucleation (two nuclei per cell, Figs 268 and 269) or multi/micronucleation (more than two nuclei per cell, Fig. 270). These appearances probably have different origins (Meriano et al., 2004). Multinucleated embryos are usually excluded from transfer. However, it has been shown that binucleated cells on Day 1 can cleave into chromosomally normal cells (Staessen and Van Steirteghem, 1998). On the other hand, severe multinucleation is probably not compatible with normal cell cleavage.
Multinucleation evaluation should be included in any embryo assessment protocol to select the highest quality embryo for transfer, and although these embryos do give rise to live births, they should be excluded from selection for embryo transfer if an alternative embryo is available.
The absence or presence of a single nucleus per blastomere has been shown to be a predictor of embryo implantation potential (Moriwaki et al., 2004; Saldeen and Sundström, 2005). Visualization of four mononucleated blastomeres in a 4-cell embryo (Figs 265 and 266) predicted a higher implantation rate than in cases where zero (Fig. 266) to three mononucleated blastomeres (Fig. 268) were seen (Saldeen and Sundström, 2005). However, other studies have found that grading embryo nuclear score on Day 2 had no additive value for the prediction of implantation rate above that predicted by Day 3 embryo morphology (Bar-Yoseph et al., 2011).
Article references:
Agerholm IE, Hnida C, Crüger DG, Berg C, Bruun-Petersen G, Kølvraa S, Ziebe S. Nuclei size in relation to nuclear status and aneuploidy rate for 13 chromosomes in donated four cells embryos. J Assist Reprod Genet 2008;25:95-102.
CrossRef | Medline | Web of Science | Google Scholar
Bar-Yoseph H, Levy A, Sonin Y, Alboteanu S, Livitas E, Lunenfeld E, Har-Vardi I. Morphological embryo assessment: reevaluation. Fertil Steril 2011;95:1624-1628.
CrossRef | Medline | Google Scholar
Hardarson T, Hanson C, Sjögren A, Lundin K. Human embryos with unevenly sized blastomeres have lower pregnancy and implantation rates: indications for aneuploidy and multinucleation. Hum Reprod 2001;16:313-318.
Abstract/FREE Full Text
Jackson KV, Ginsburg ES, Hornstein MD, Rein MS, Clarke RN. Multinucleation in normally fertilized embryos is associated with an accelerated ovulation induction response and lower implantation and pregnancy rates in in vitro fertilization-embryo transfer cycles. Fertil Steril 1998;70:60-66.
CrossRef | Medline | Web of Science | Google Scholar
Meriano J, Clark C, Cadesky K, Laskin CA. Binucleated and multinucleated blastomeres in embryos derived from human assisted reproduction cycles. Reprod BioMed Online 2004;9:511-520.
Medline | Web of Science | Google Scholar
Moriwaki T, Suganuma N, Hayakawa M, Hibi H, Katsumata Y, Oguchi H, Furuhashi M. Embryo evaluation by analysing blastomere nuclei. Hum Reprod 2004;19:152-156.
Abstract/FREE Full Text
Pelinck M, De Vos M, Dekens M, Van der Elst J, De Sutter P, Dhont M. Embryos cultured in vitro with multinucleated blastomeres have poor implantation potential in human in-vitro fertilization and intracytoplasmic sperm injection. Hum Reprod 1998;13:960-963.
Abstract/FREE Full Text
Pickering SJ, Taylor A, Johnson MH, Braude PR. Diagnosing and preventing inherited disease: an analysis of multinucleated blastomere formation in human embryos. Hum Reprod 1995;10:1912-1922.
Abstract/FREE Full Text
Saldeen P, Sundström P. Nuclear status of four cell preembryos predicts implantation potential in IVF treatment cycles. Fertil Steril 2005;84:584-589.
CrossRef | Medline | Web of Science | Google Scholar
Staessen C, Van Steirteghem A. The genetic constitution of multinuclear blastomeres and their derivative daughter blastomeres. Hum Reprod 1998;13:1625-1631.
Abstract/FREE Full Text
Van Royen E, Mangelschots K, Vercruyssen M, De Neubourg D, Valkenburg M, Ryckaert G, Gerris J. Multinucleation in cleavage stage embryos. Hum Reprod 2003;18:1062-1069.
Abstract/FREE Full Text
Ziebe S, Lundin K, Loft A, Bergh C, Nyboe Andersen A, Selleskog U, Nielsen D, Grøndahl C, Kim H, Arce JC; for the CEMAS II and III Study Group. FISH analysis for chromosomes 13, 16, 18, 21, 22, X and Y in all blastomeres of IVF pre-embryos from 144 randomly selected donated human oocytes and impact on pre-embryo morphology. Hum Reprod 2003;18:2575-2581.
Abstract/FREE Full Text