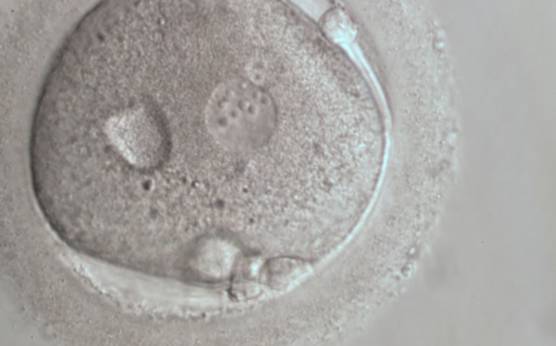
B. Pronuclear size
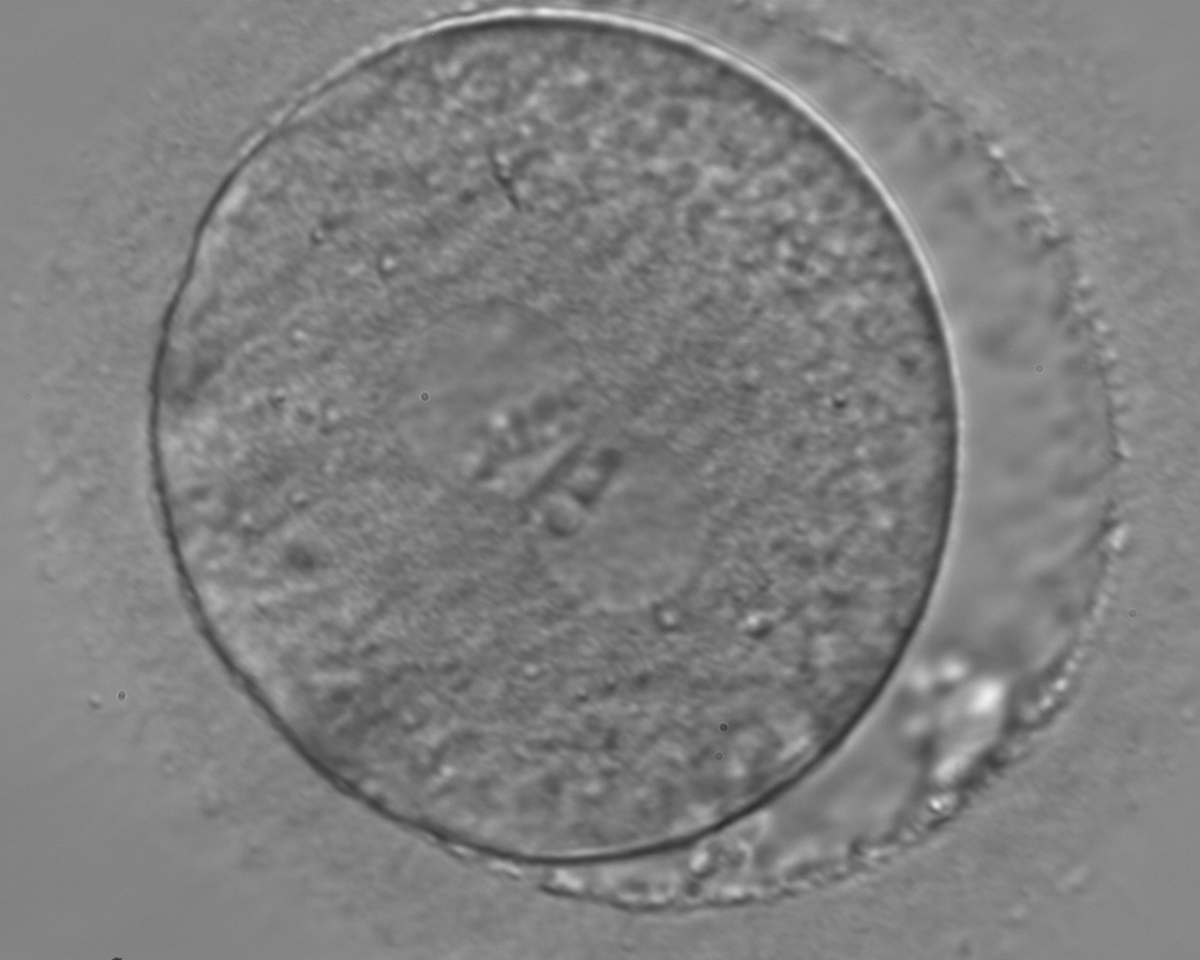
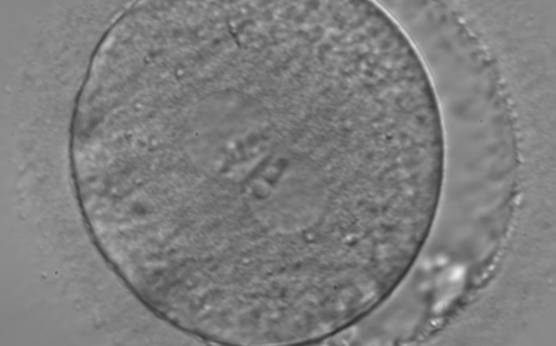
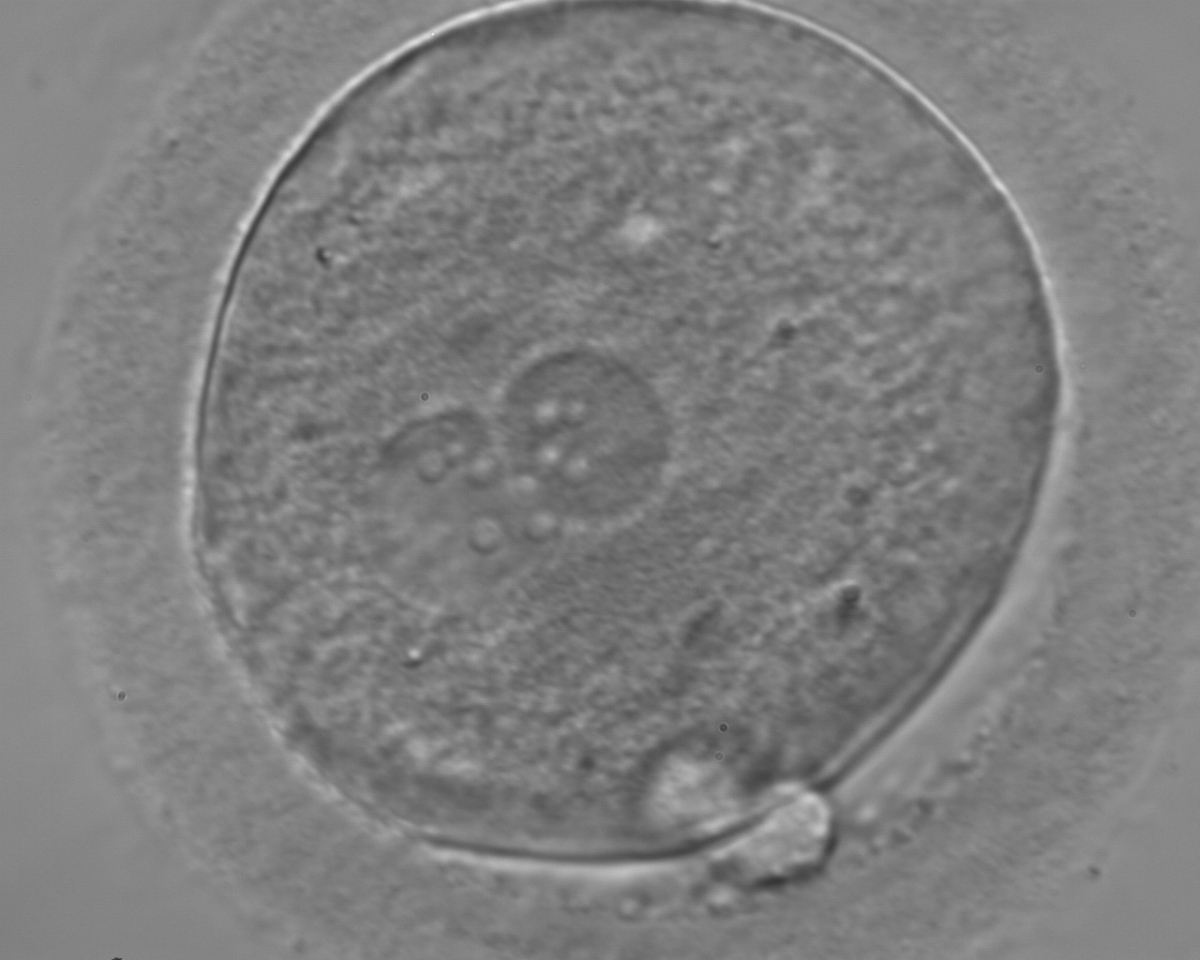
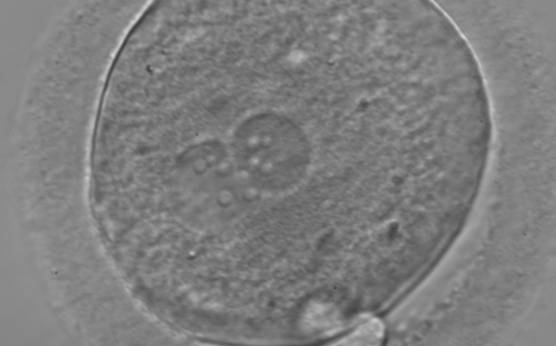
B.1 Normal
Some studies have demonstrated that the size of PNs depends on gamete factors. Decondensation of the tightly compacted sperm chromatin is a crucial step in fertilization that includes the replacement of protamines with histones under the control of oocyte-decondensing factors. Therefore, the male PN size depends both on the sperm nuclear structure and on the oocyte's capacity of inducing decondensation by releasing appropriate levels of glutathione.
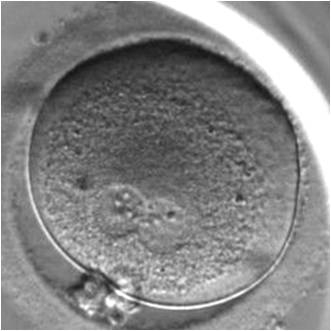
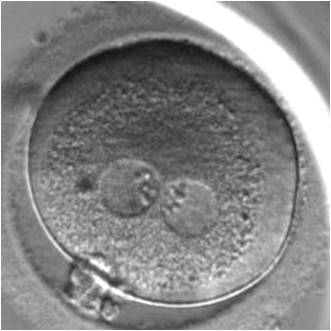
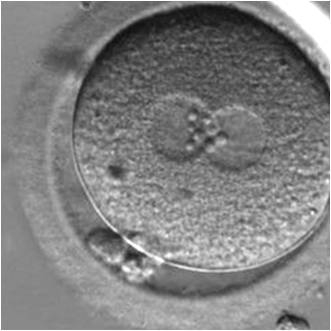
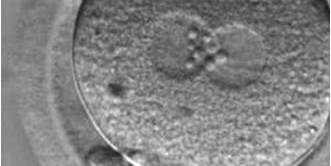
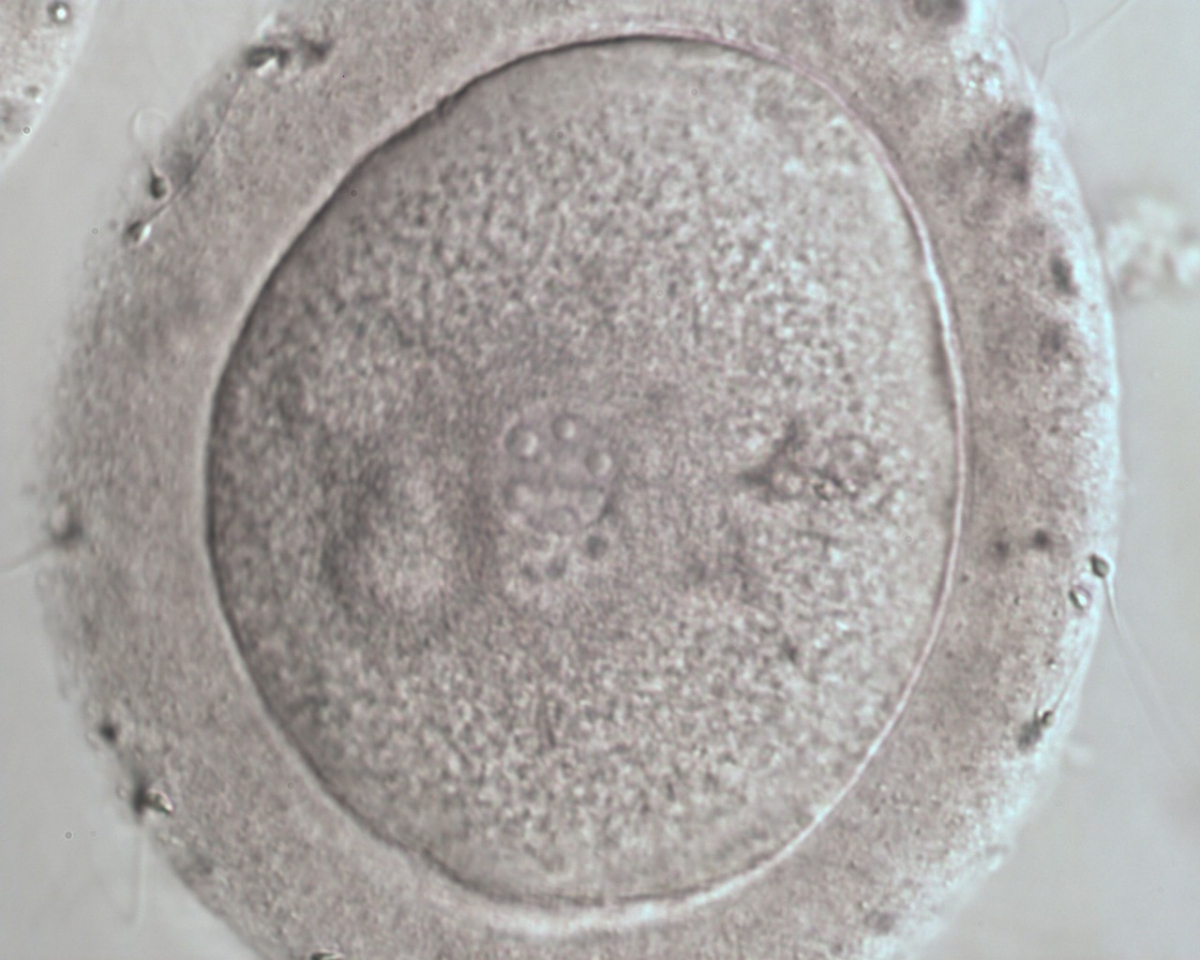
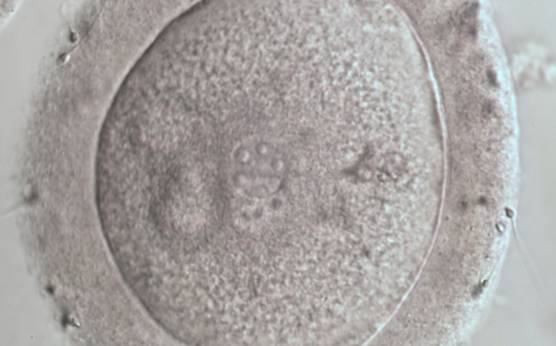
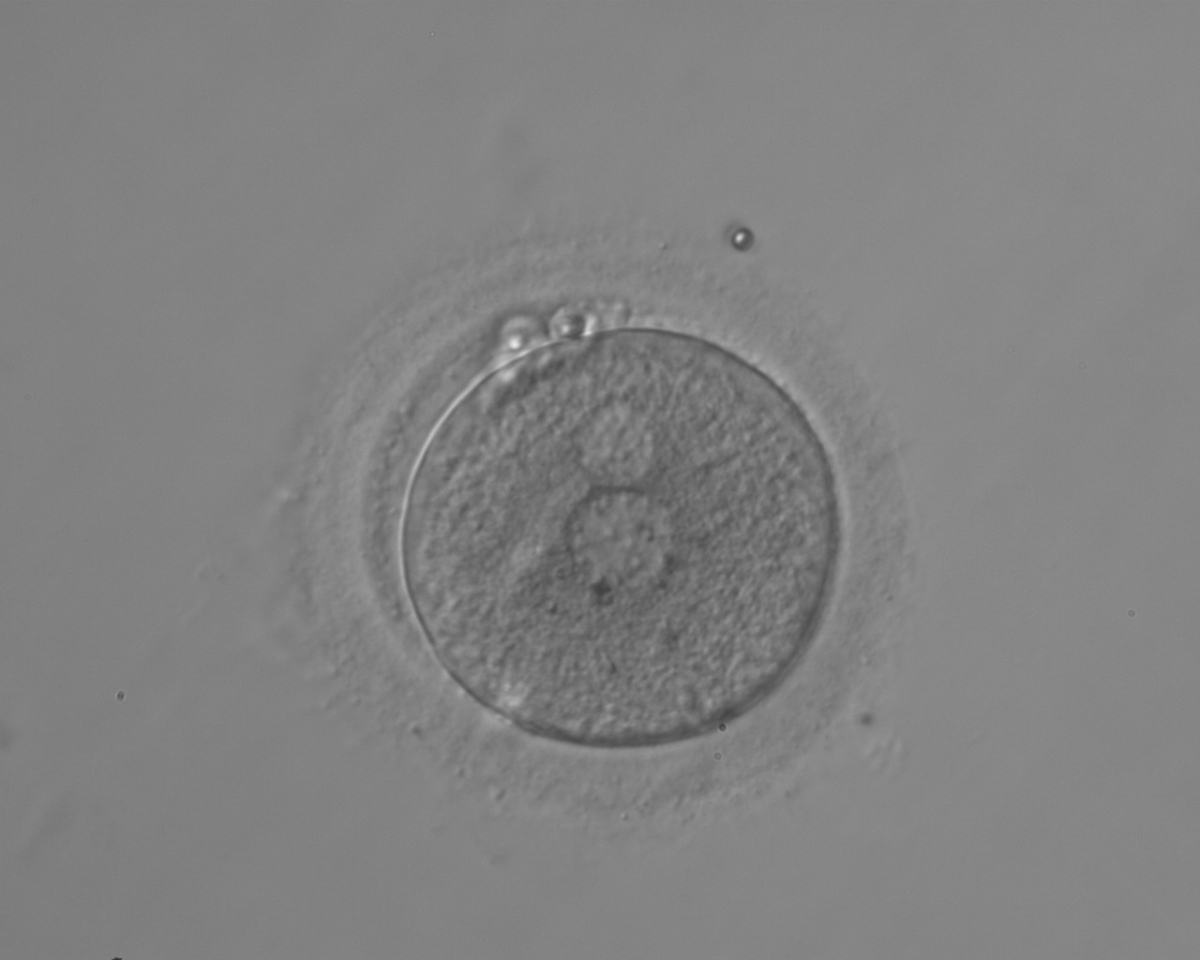
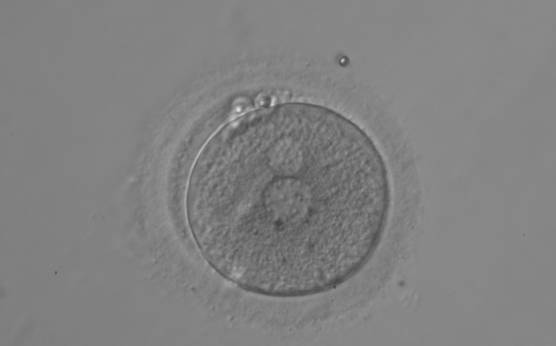
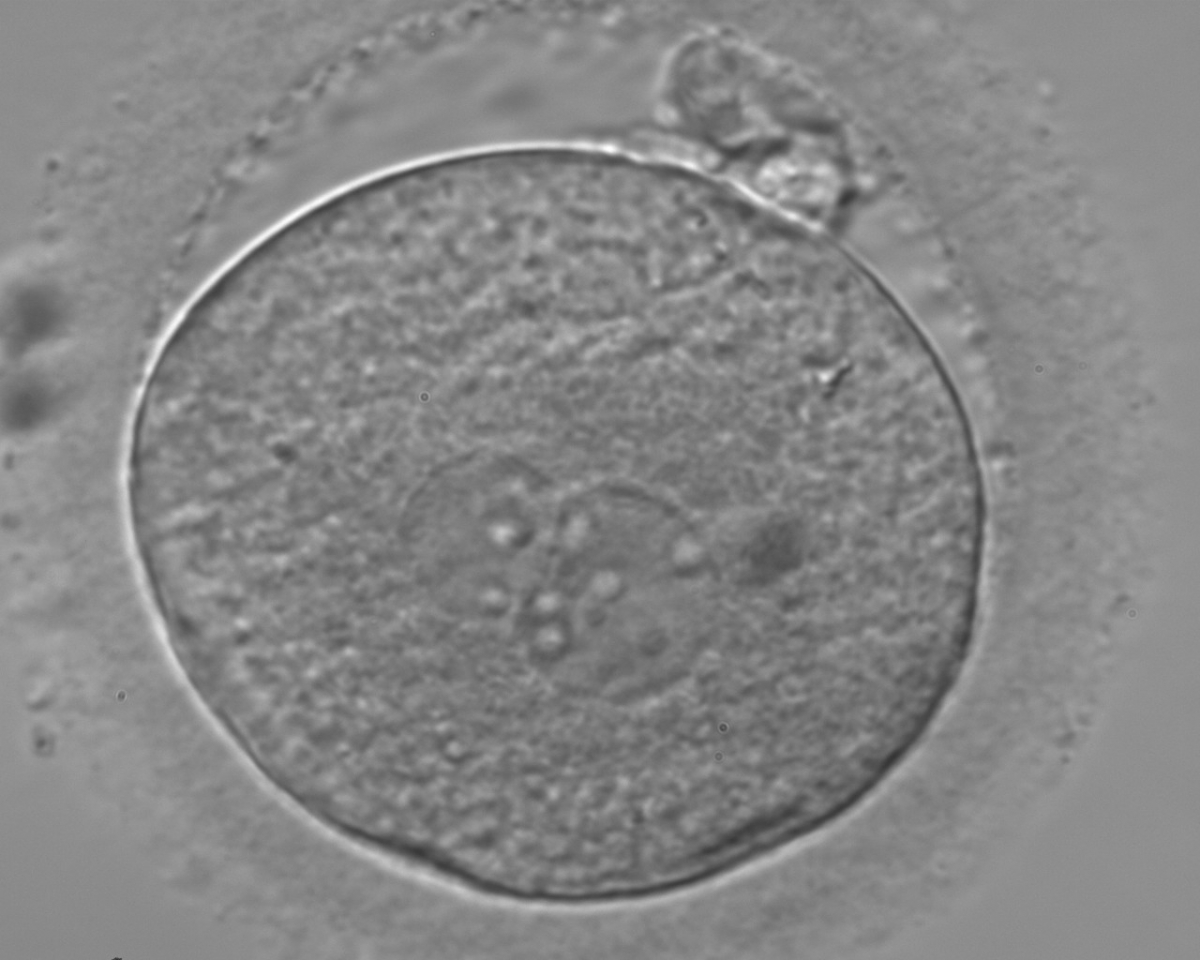
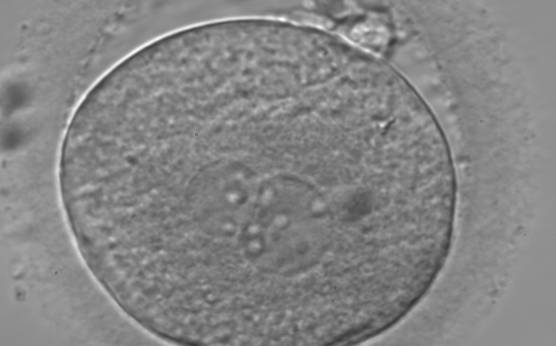
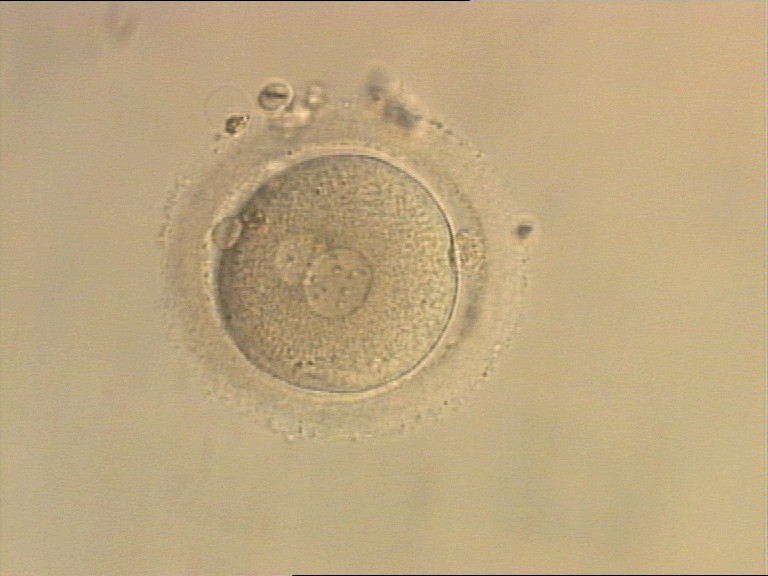
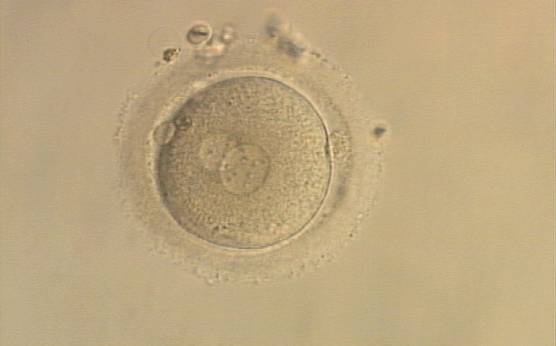
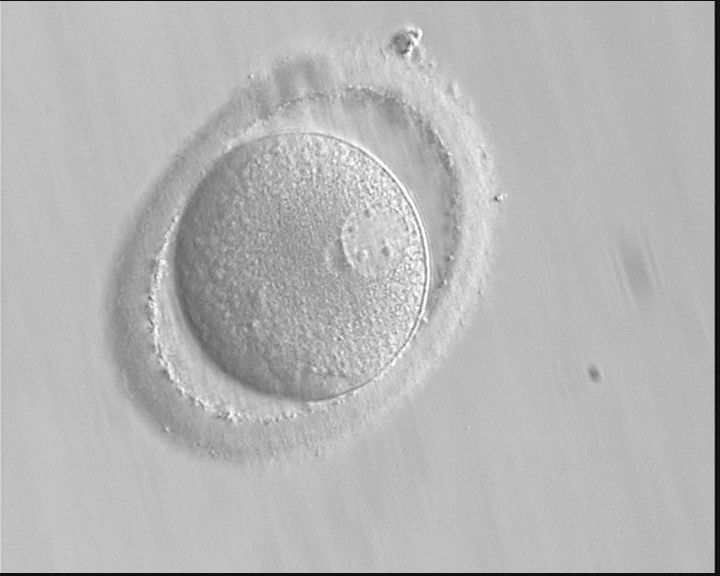
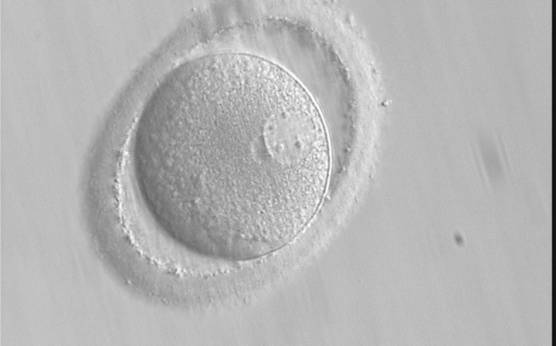
PNs normally appear to be similar in size, although the female PN that is often located towards the second polar body can be slightly smaller (Figs 99 and 100). However, PN formation and rotation is a dynamic process as demonstrated by time-lapse video recording, so positioning and morphology of PNs are strictly time dependent (Fig. 101).
B.2 Small
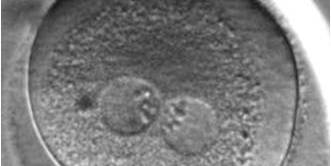
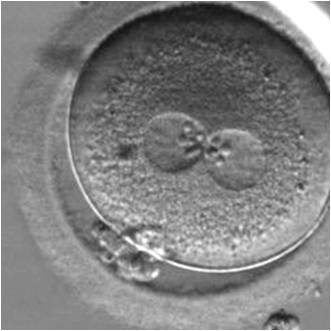
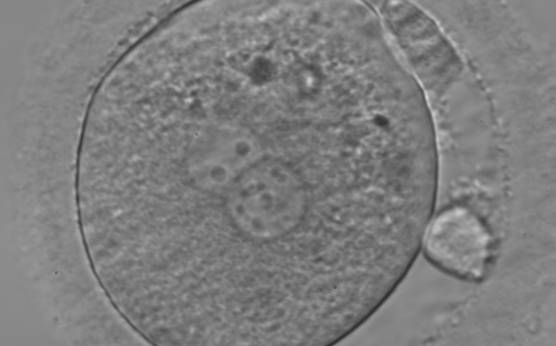
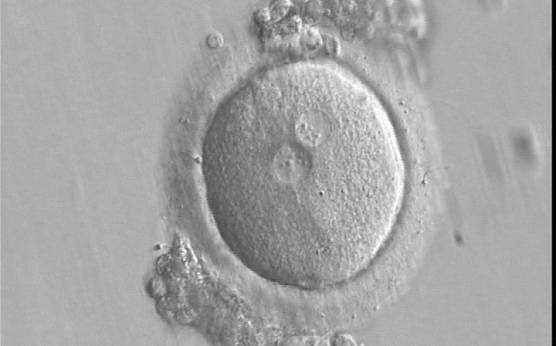
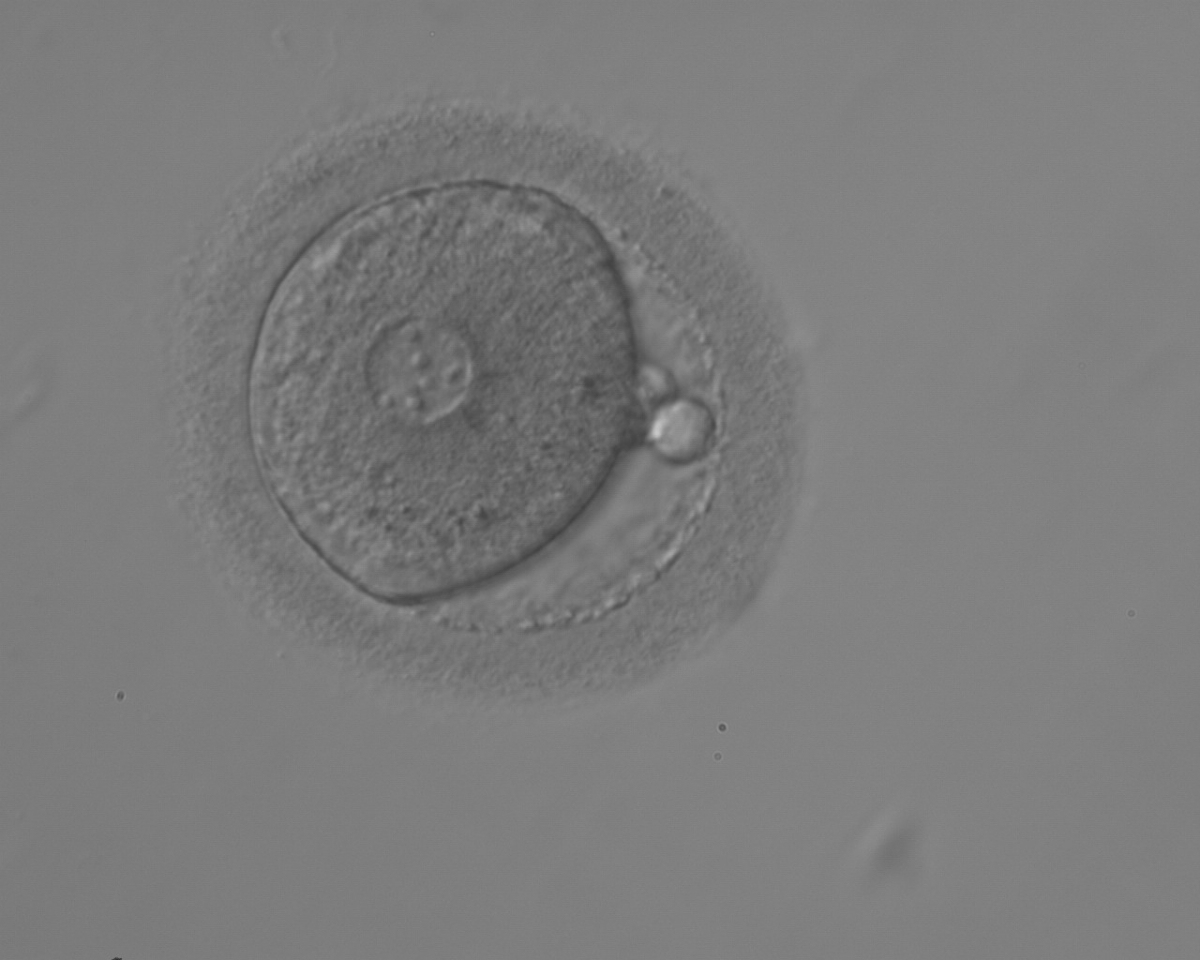
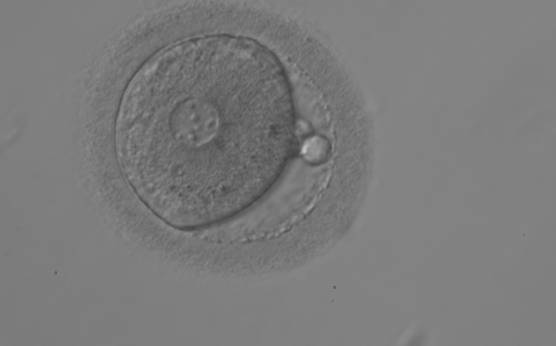
The size of PNs depends on the time of observation with an increase in size of close to 50% from abuttal to 17 h post-ICSI (Payne et al., 1997). The presence of PNs with a diameter smaller than normal (Figs 102–104) at the normal time of fertilization check could be an indicator of delayed fertilization possibly due to oocyte immaturity, or defects in the gametes. Nevertheless, implantation can occur as a result of transfer of these zygotes (Fig. 103).
B.3 Differential in size
Significant differences (>4 μm) between PN size (Figs 105–108), or the presence of micronuclei or fragmented PNs (Figs 109 and 110) are considered to be abnormal and are associated with chromosomal abnormalities and major loss of developmental potential (Munné and Cohen, 1998; Scott et al., 2000; Nagy et al., 2003; Scott et al., 2007; Alpha Scientists in Reproductive medicine and ESHRE Special Interest Group of Embryology, 2011). Clinical studies have shown a high correlation between zygotes observed at 16–18 h post-insemination displaying large differences in PN size and their capability to maintain viability and development both in vivo and in vitro (Sadowy et al., 1998; Scott et al., 2000). Therefore, when performing embryo selection these embryos should be avoided for transfer.
Article references:
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 2011;26:1270-1283.
Abstract/FREE Full Text
Munné S, Cohen J. Chromosome abnormalities in human embryos. Hum Reprod Update 1998;4:842-855.
Abstract/FREE Full Text
Nagy ZP, Dozortsev D, Diamond M, Rienzi L, Ubaldi F, Abdelmassih R, Greco E. Pronuclear morphology evaluation with subsequent evaluation of embryo morphology significantly increases implantation rates. Fertil Steril 2003;80:67-74.
Medline | Web of Science | Google Scholar
Payne D, Flaherty SP, Barry MF, Matthews CD. Preliminary observations on polar body extrusion and pronuclear formation in human oocytes using time-lapse video cinematography. Hum Reprod 1997;12:532-541.
Abstract/FREE Full Text
Sadowy S, Tomkin G, Munné S, Ferrara-Congedo T, Cohen J. Impaired development of zygotes with uneven pronuclear size. Zygote 1998;6:137-141.
CrossRef | Medline | Web of Science | Google Scholar
Scott L, Alvero R, Leondires M, Miller B. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod 2000;15:2394-2403.
Abstract/FREE Full Text
Scott L, Finn A, O'Leary T, McLellan S, Hill J. Morphologic parameters of early cleavage-stage embryos that correlate with fetal development and delivery: prospective and applied data for increased pregnancy rates. Hum Reprod 2007;22:230-240.
Abstract/FREE Full Text