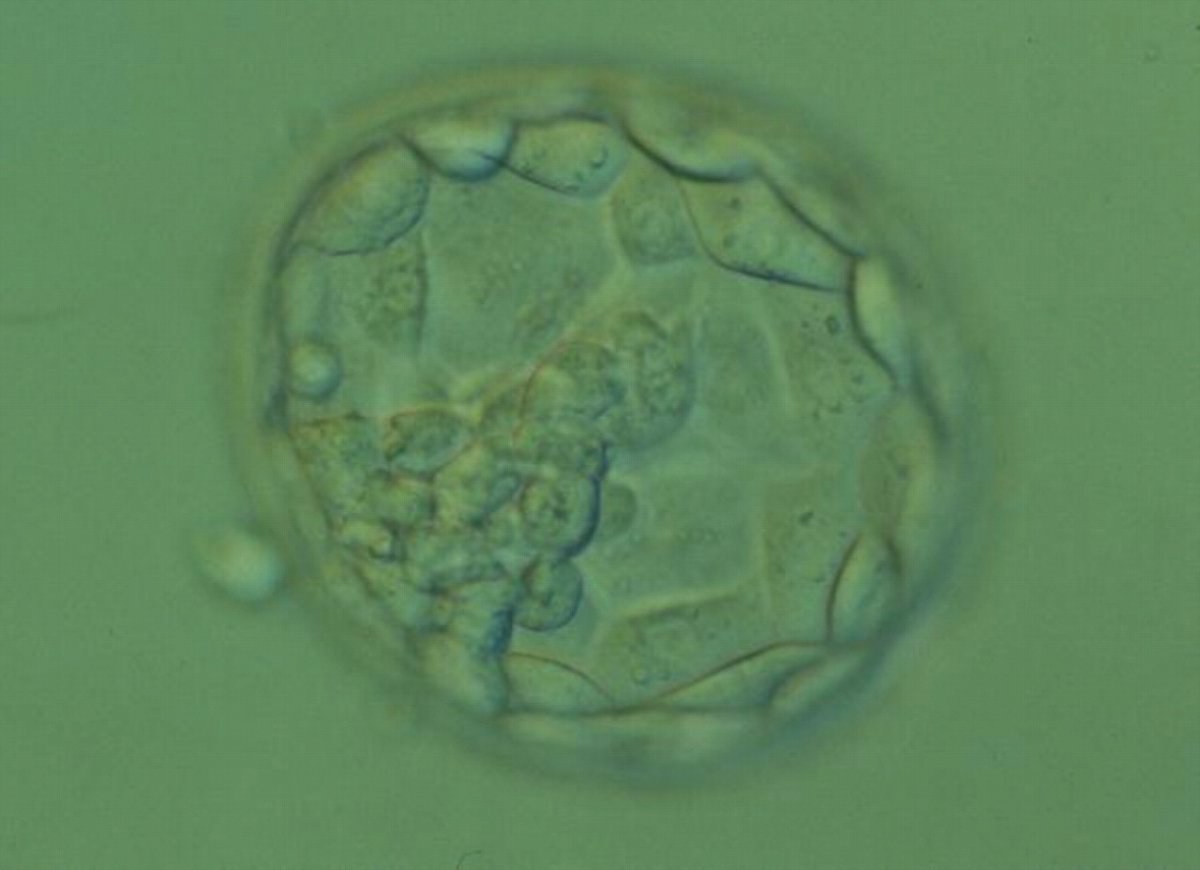
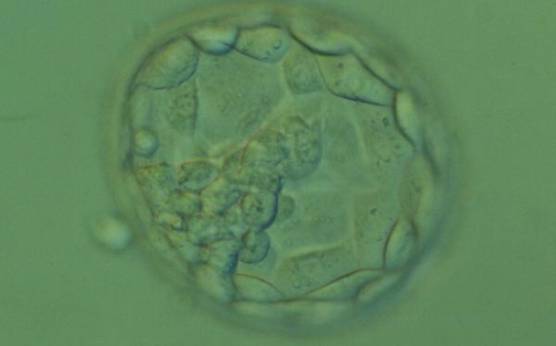
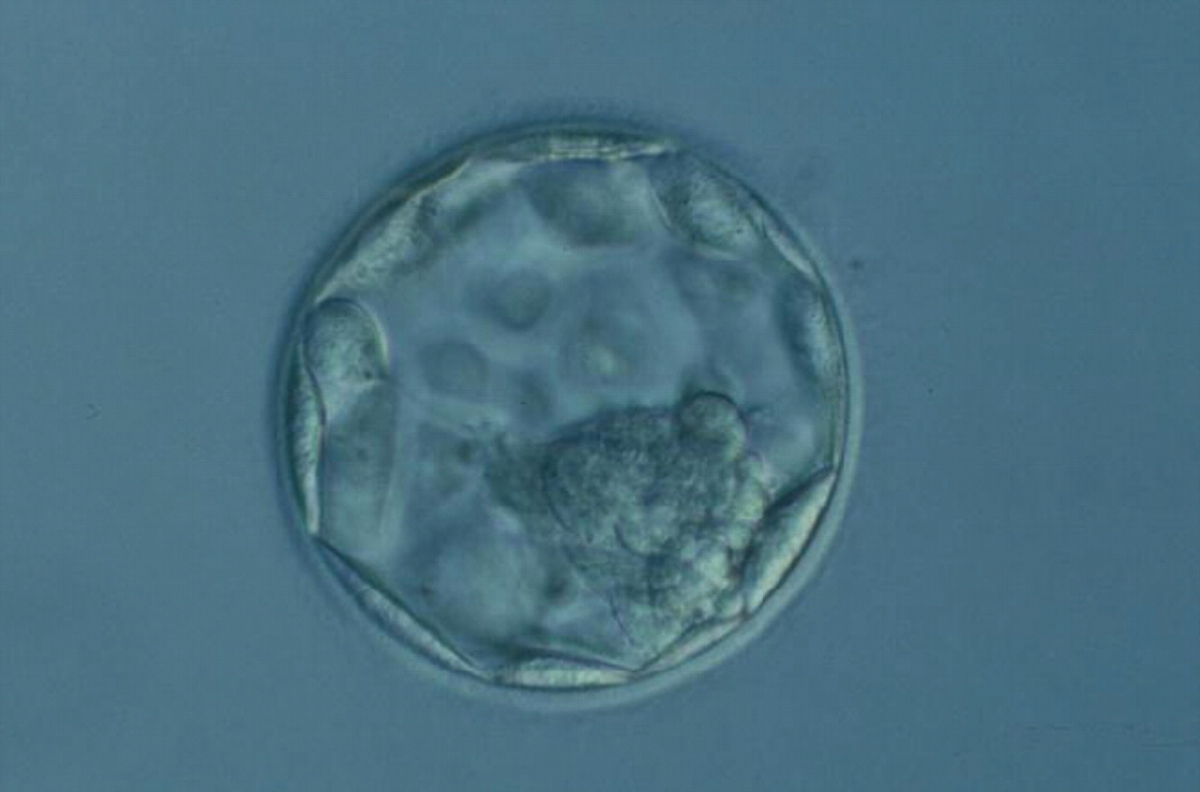
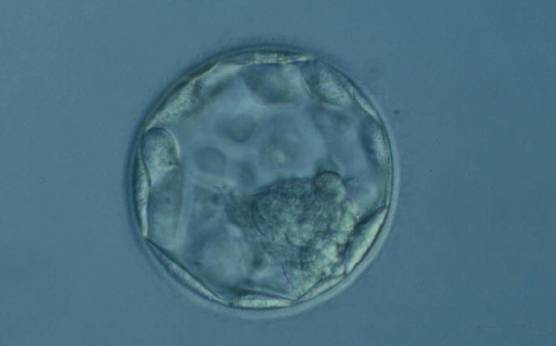
C. Trophectoderm morphology
The TE cells can be clearly distinguished from the ICM cells as the blastocyst begins to expand (i.e. expansion Grade 3 or higher). The role of the TE cells in the early stages of blastocyst development is not entirely clear but their role in creating the fluid filled blastocoel may be a key parameter in ICM determination. The role of the TE cells, however, is better understood during and after implantation as they play a key role in apposition, adhesion and invasion of the endometrium, thus allowing the blastocyst to embed in the uterus. The TE cells also produce several molecular factors that aid in the implantation process (Aplin, 2000). Without properly functional TE cells, the embryo would remain within the ZP as these cells are actively involved in breaking free of the ZP (Sathananthan et al., 2003). The ultimate fate of the TE cells is to become the fetal extra-embryonic membranes as well as the placenta.
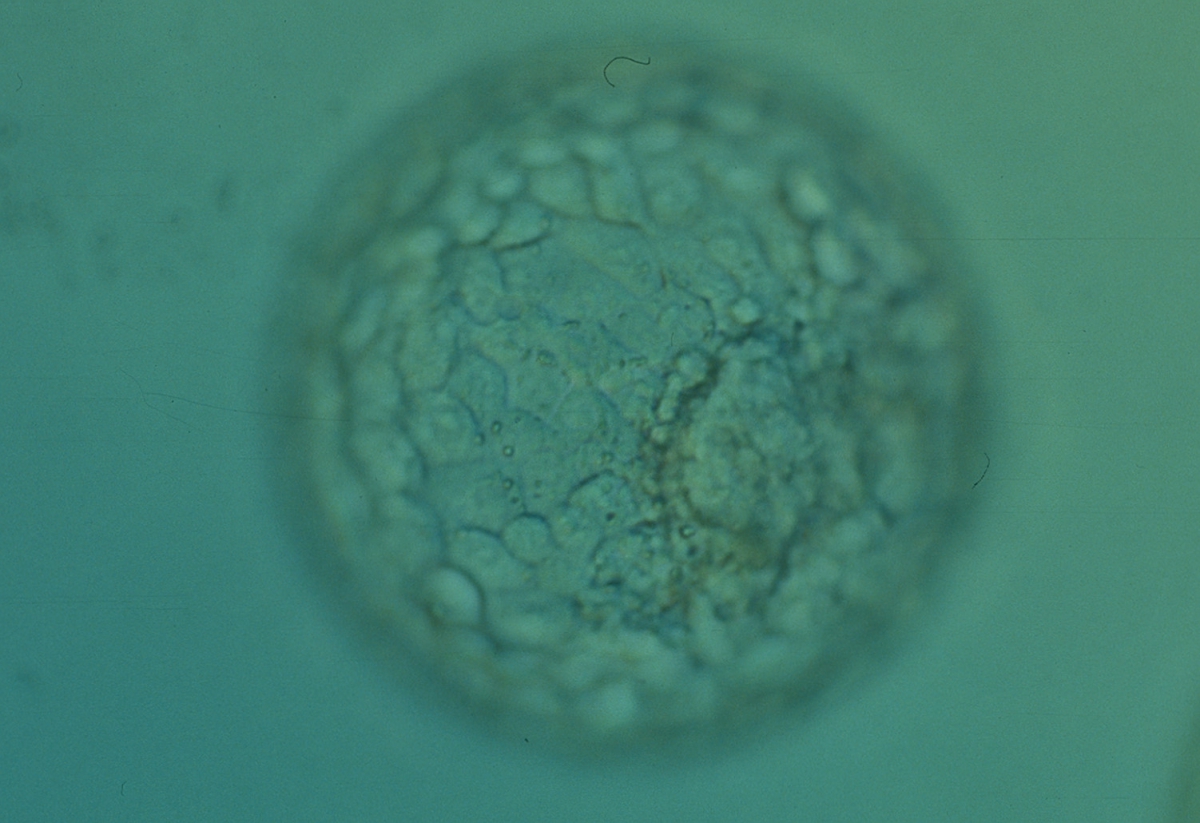
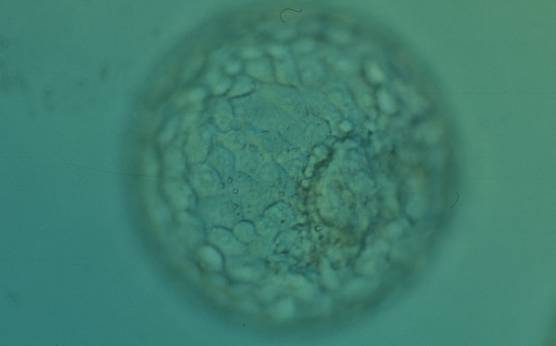
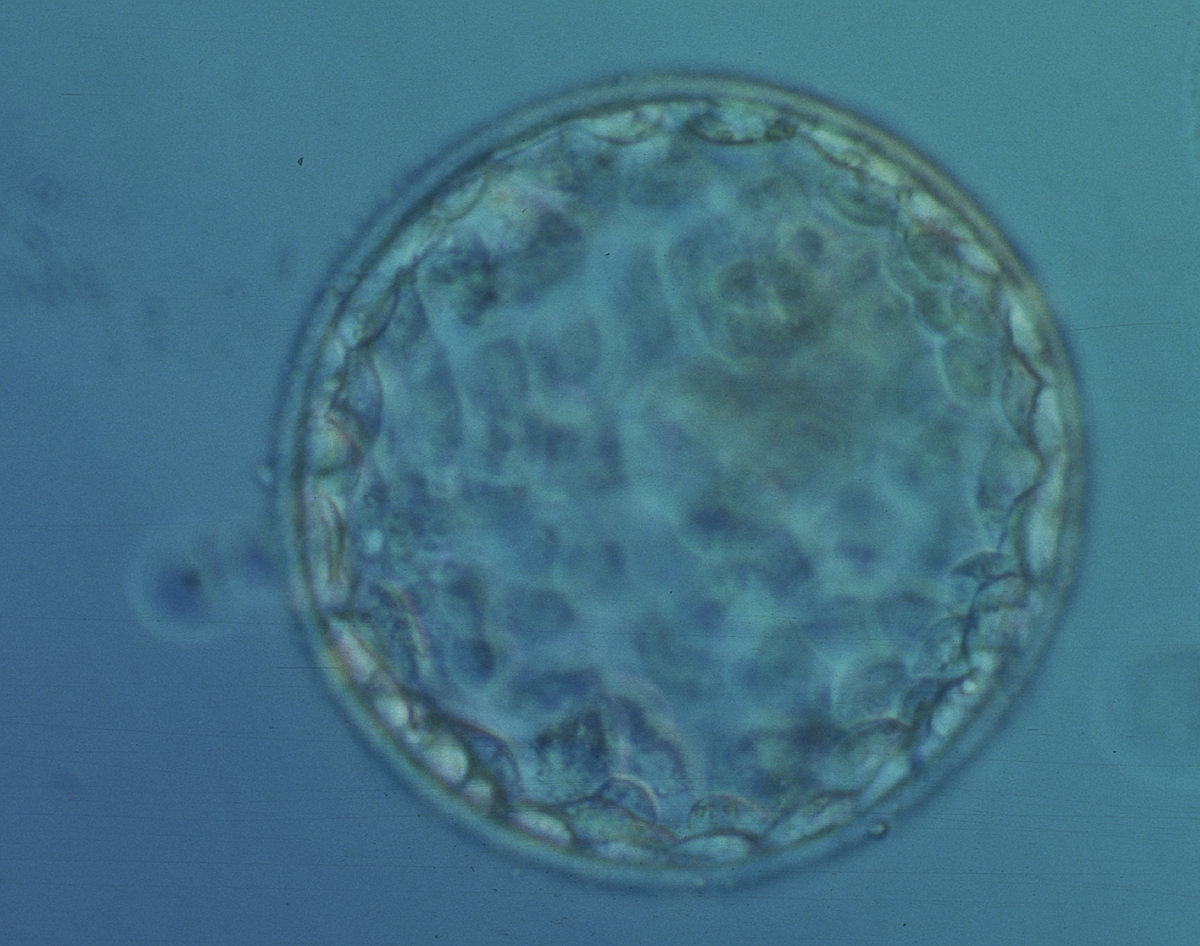
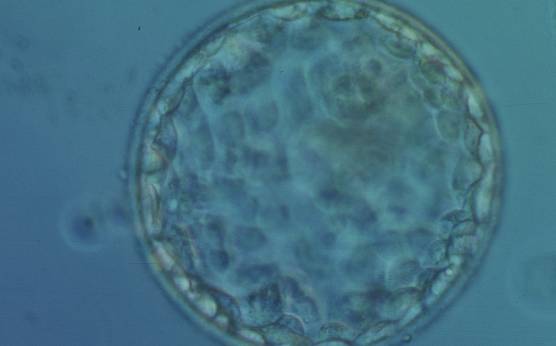
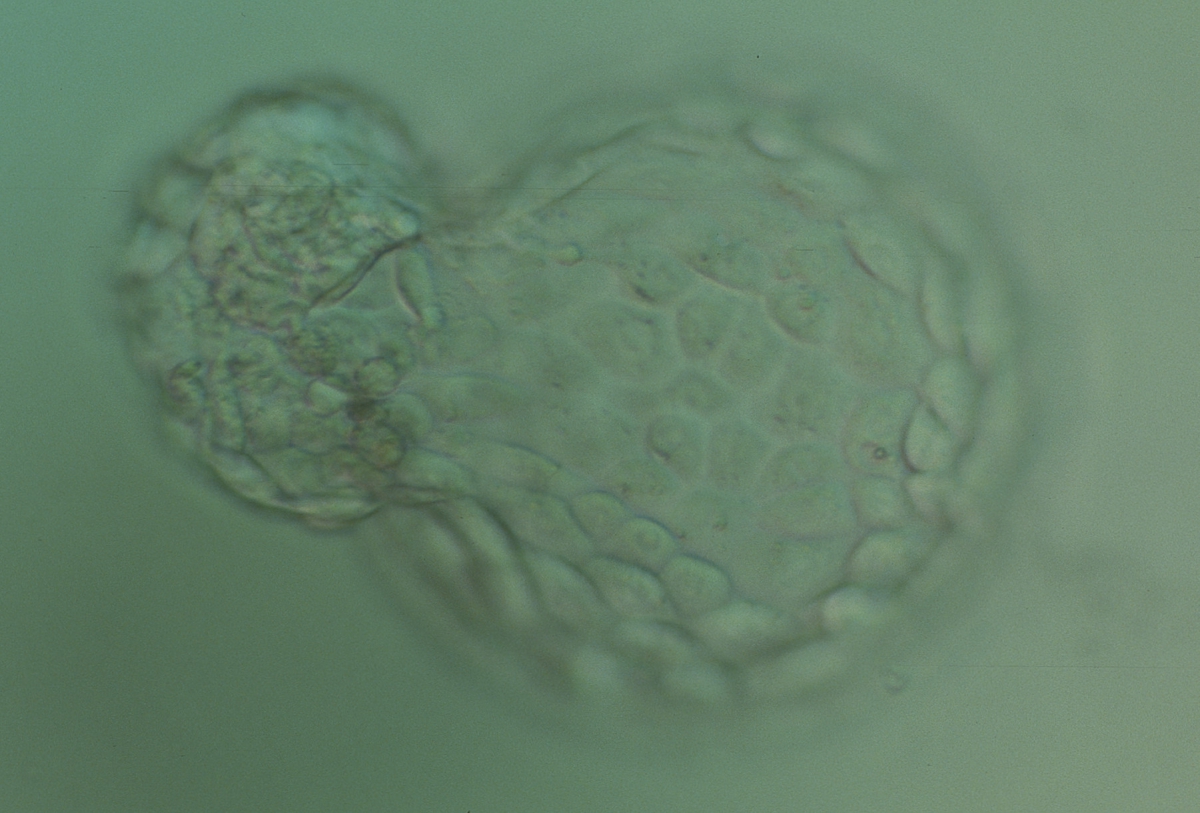
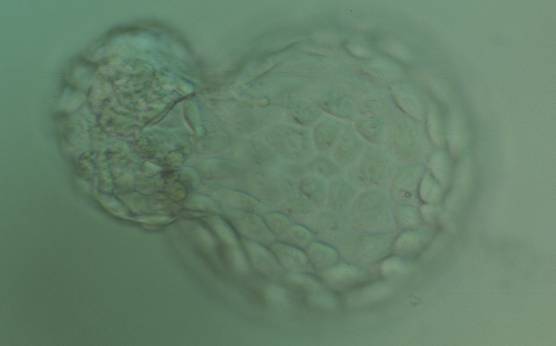
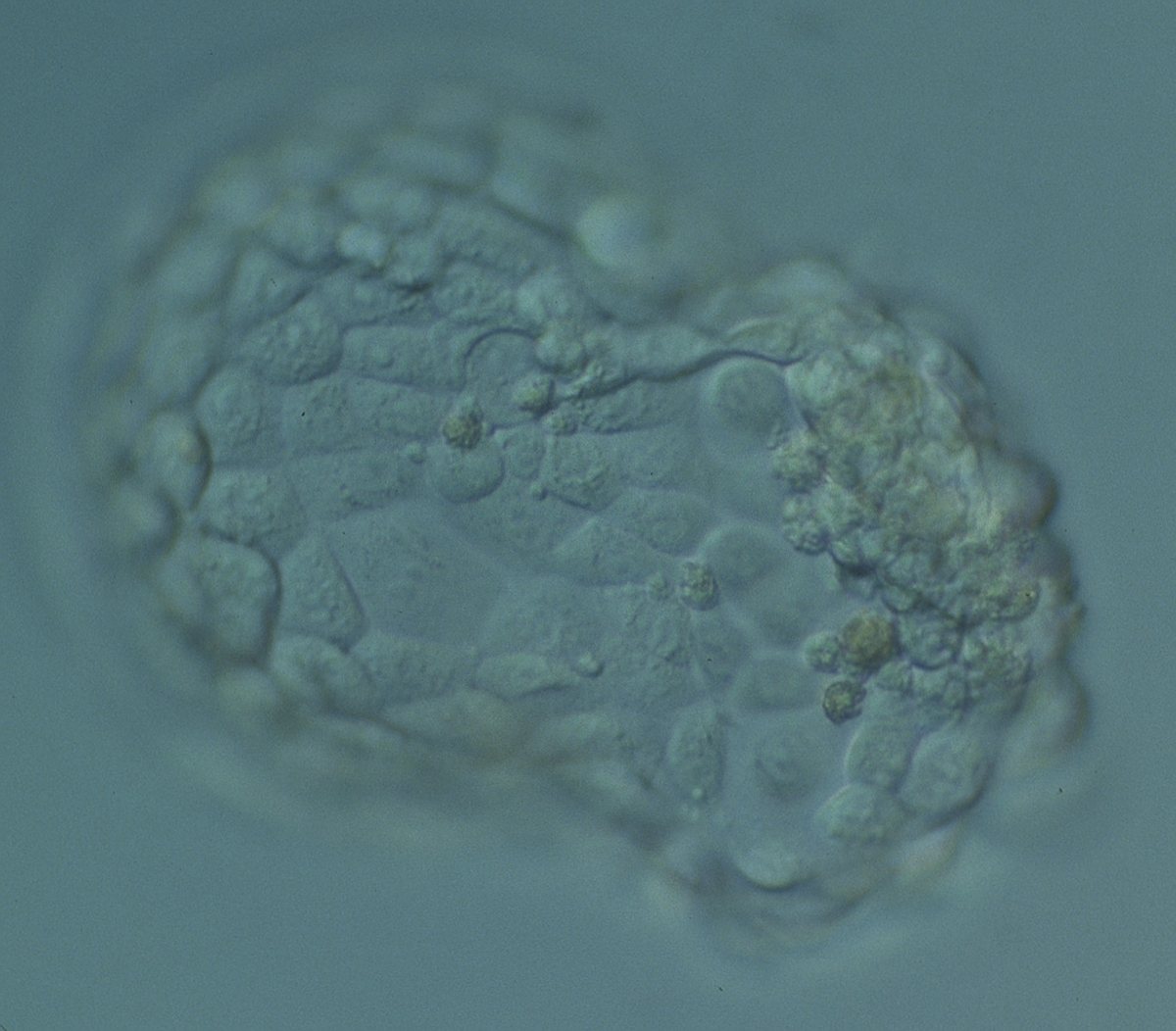
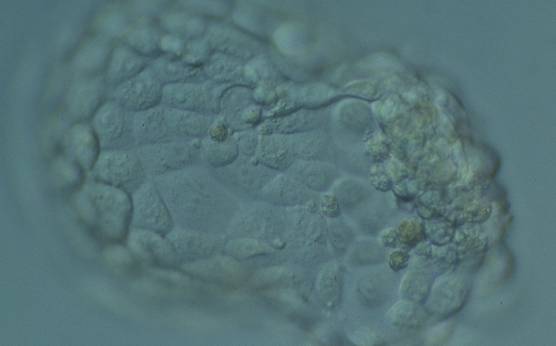
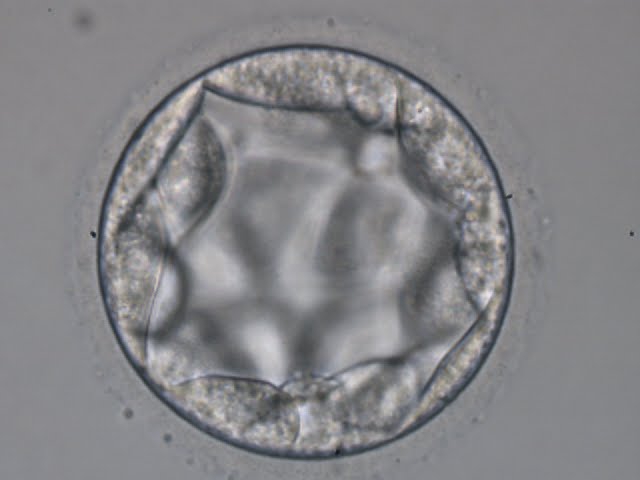
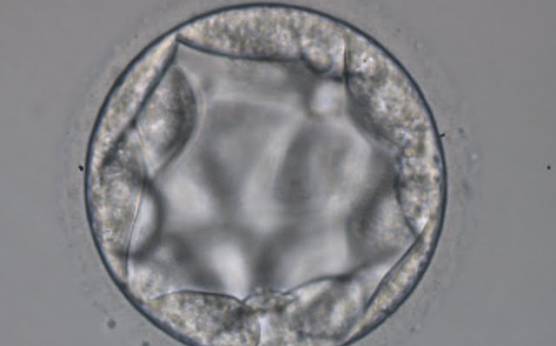
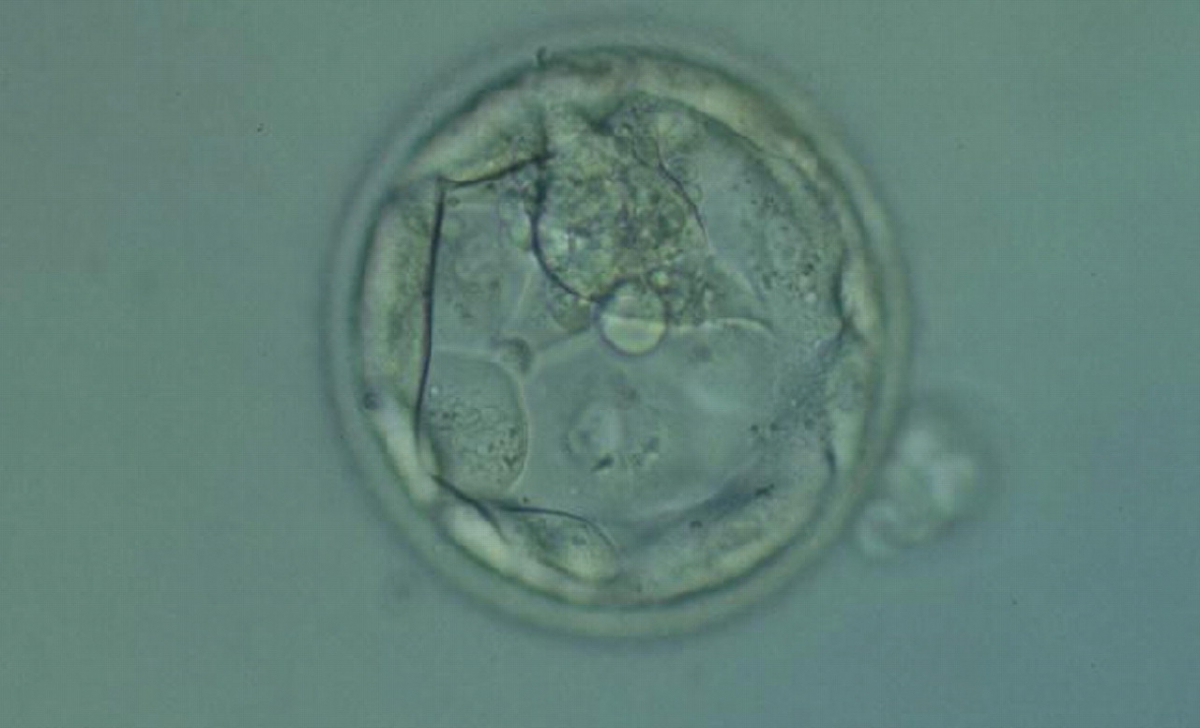
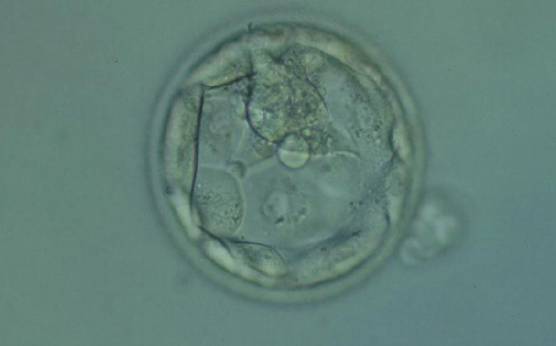
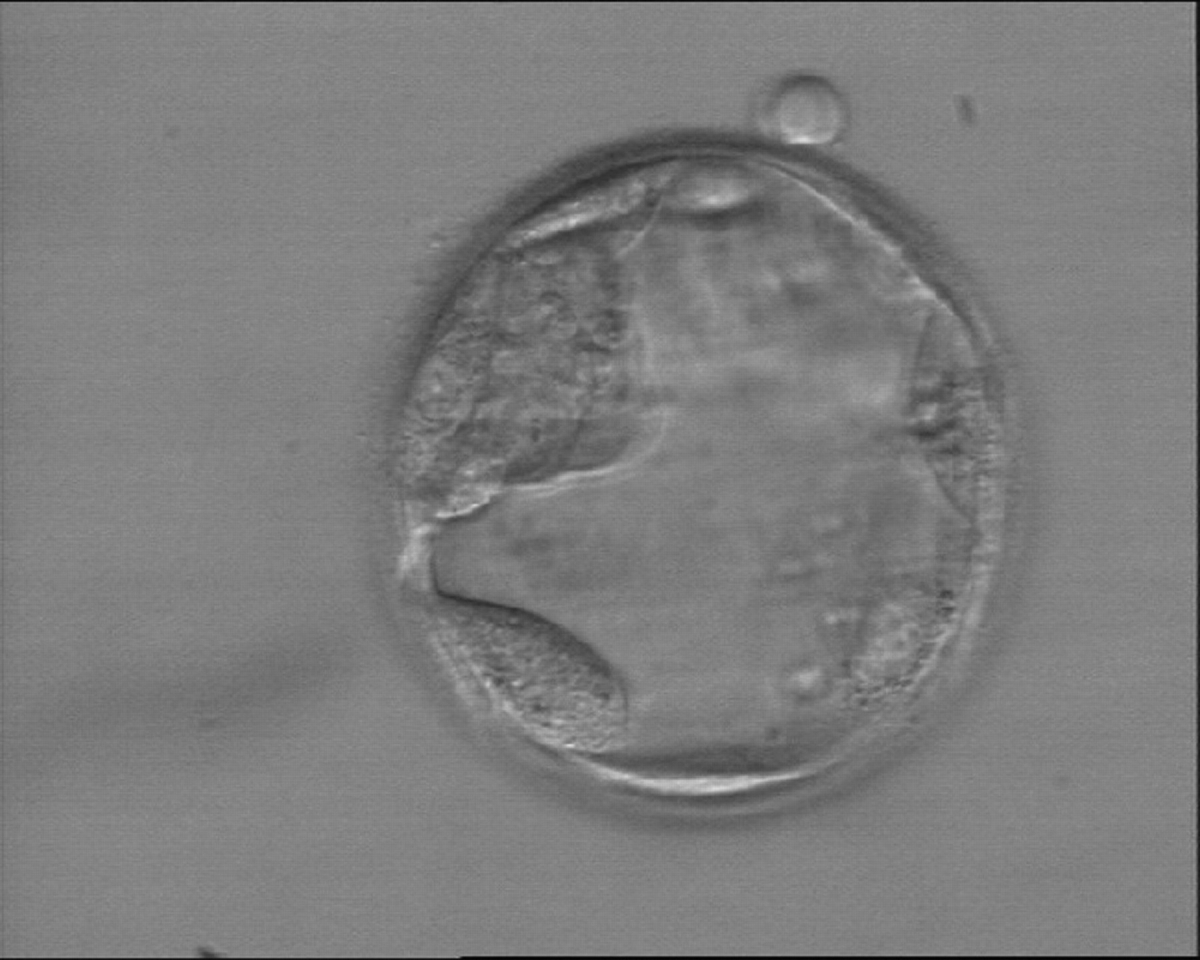
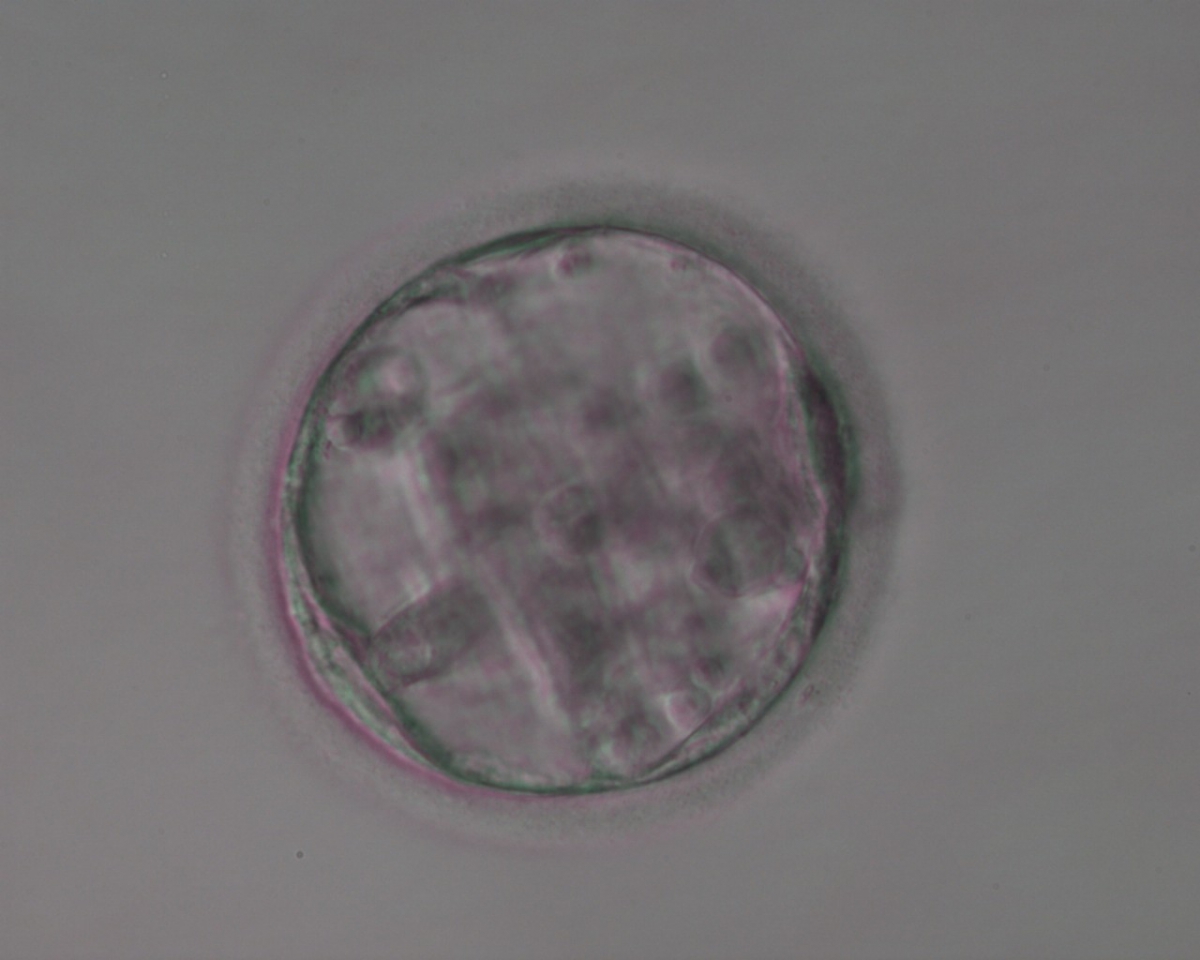
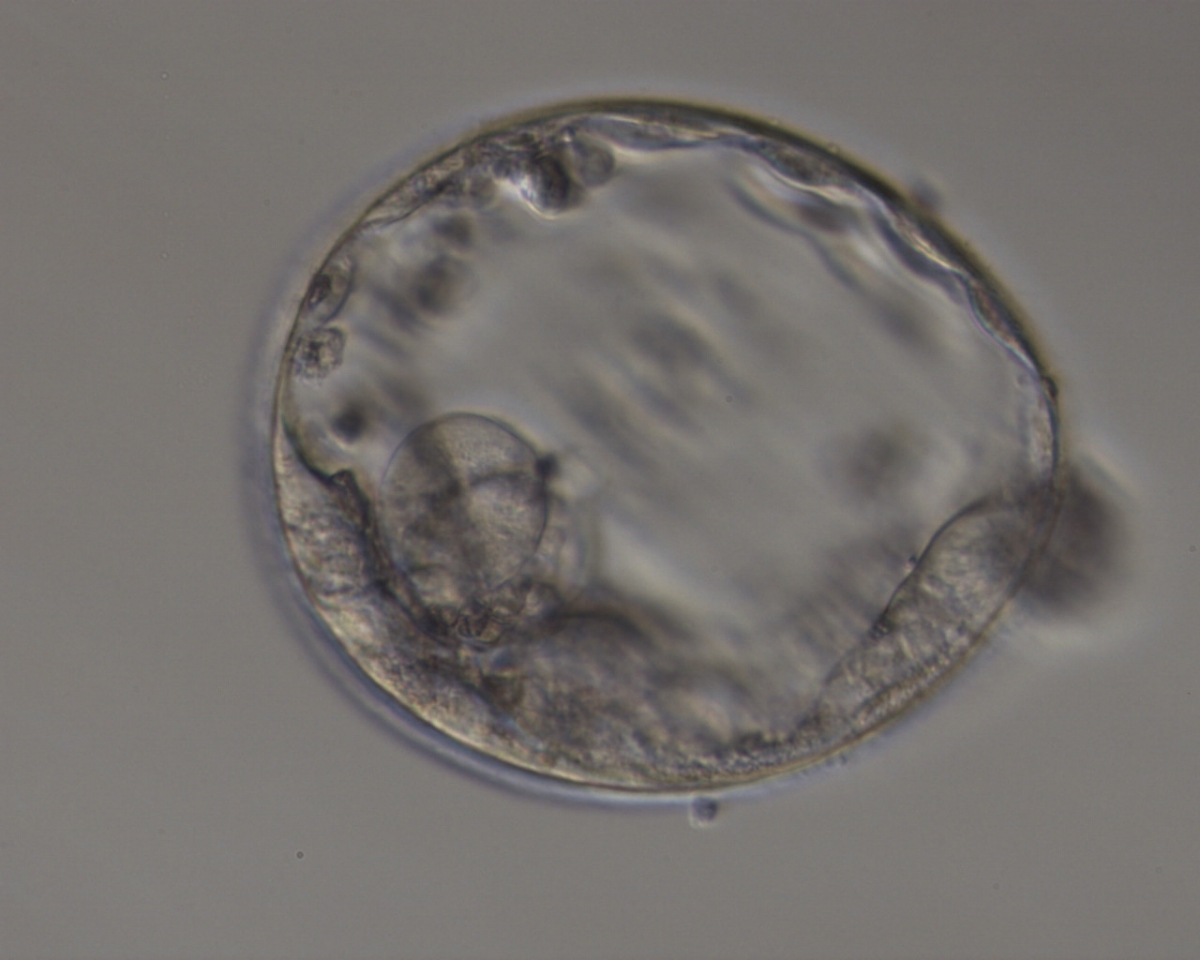
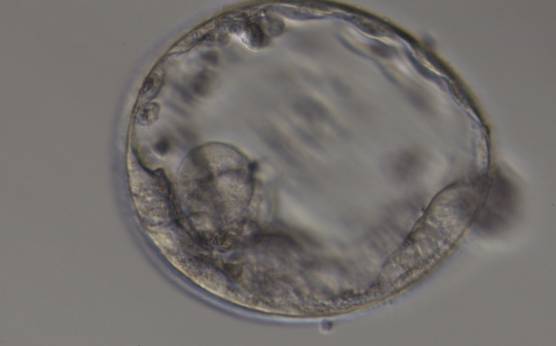
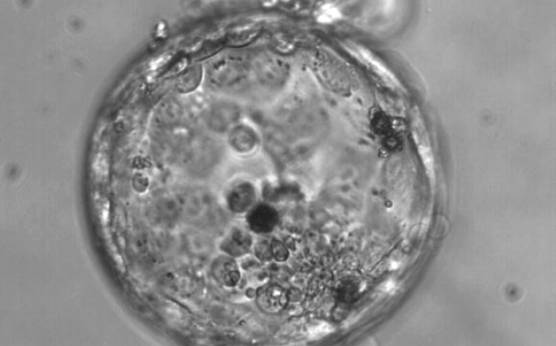
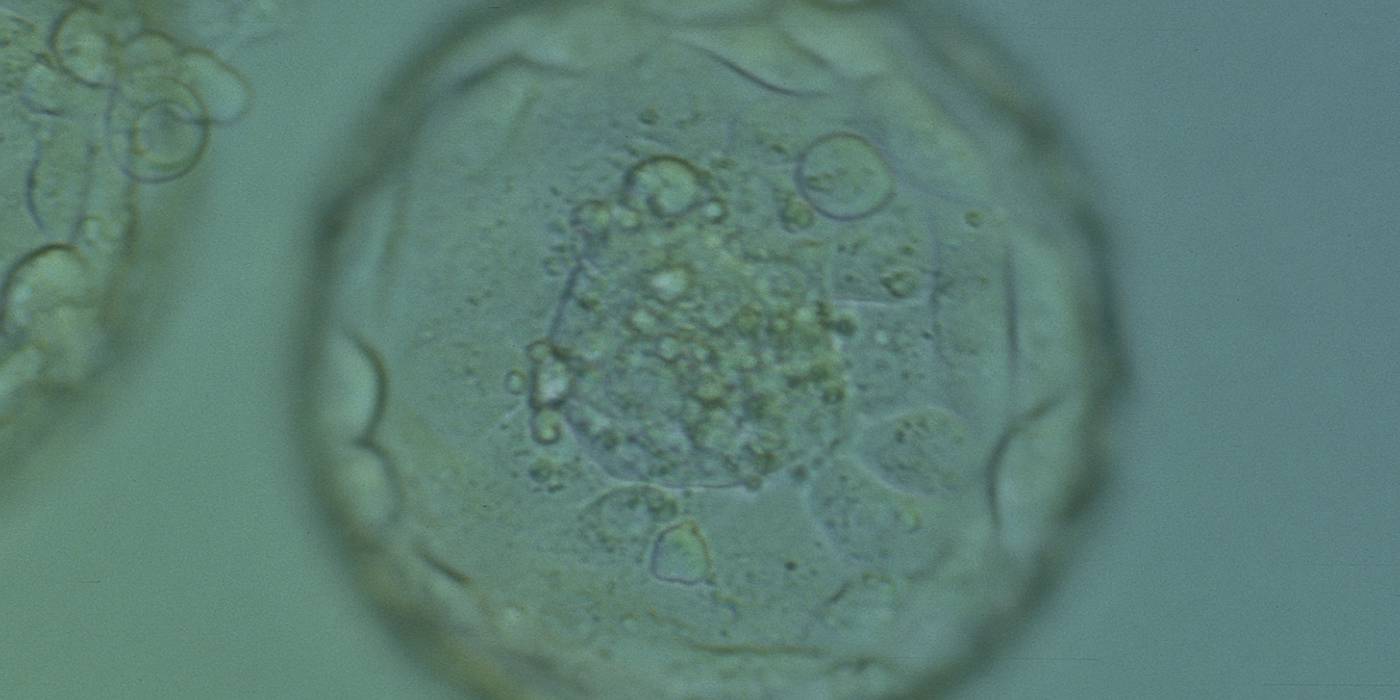
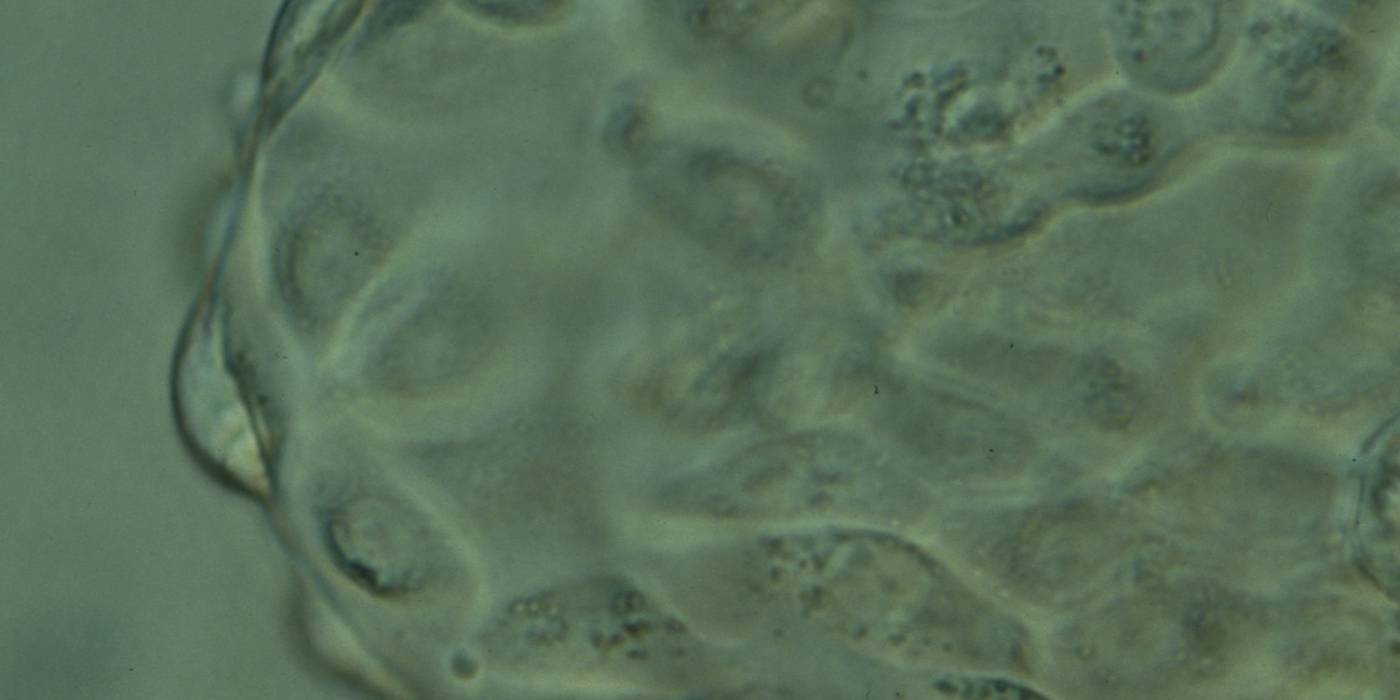
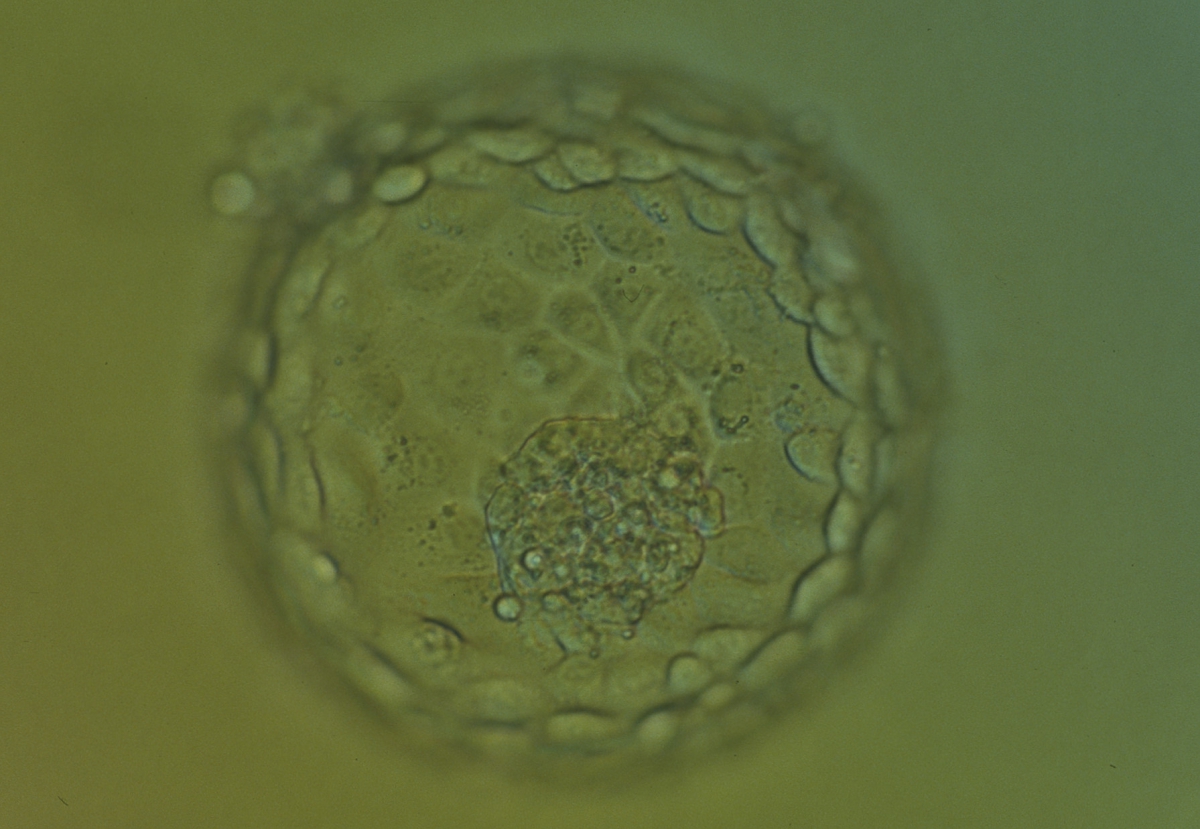
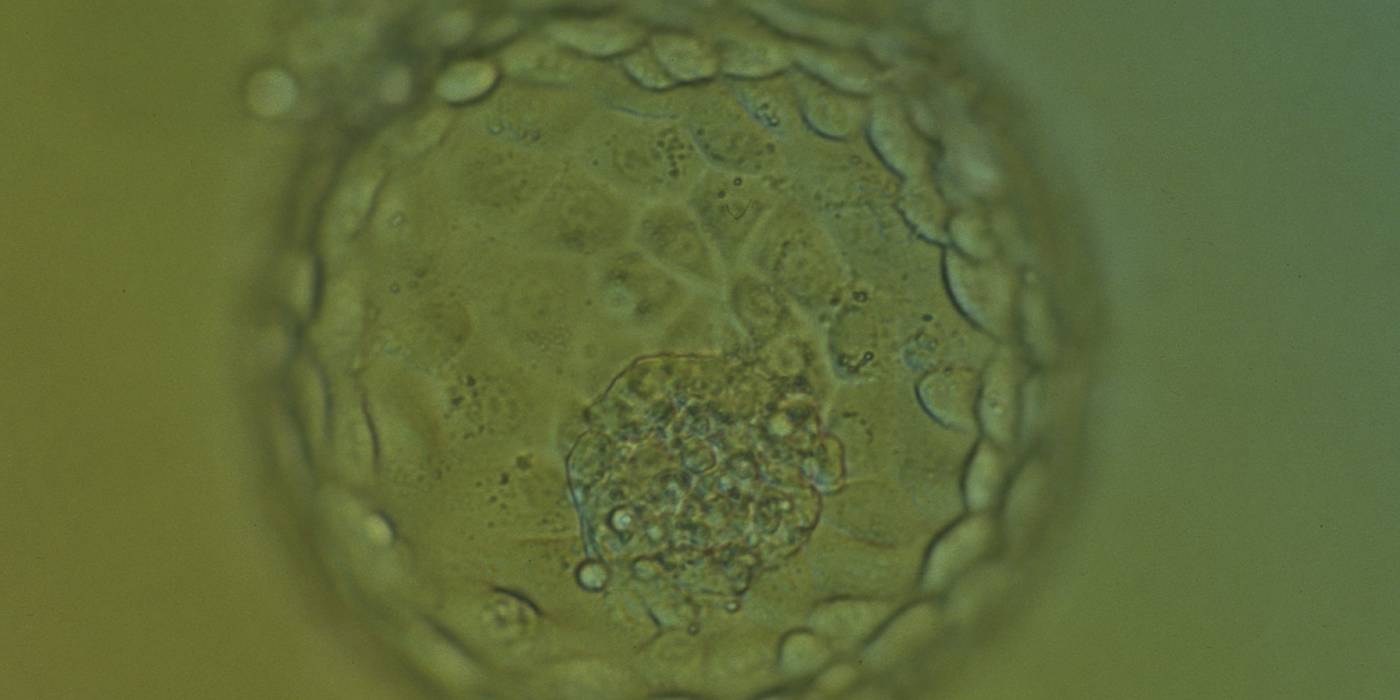
The TE cells have traditionally been scored in a similar manner to the ICM, i.e. by their number and cohesiveness according to three different grades (1–3). The best TE category (1) contains many cells that form a cohesive epithelium (Figs 365–368), the middle TE category (2) is composed of few cells forming a loose epithelium (Figs 369–372) and the worst category (3) describes a TE that contains very few, large cells that struggle to form a cohesive epithelium (Figs 373–376). The grading of the TE cells has been demonstrated in some reports to have an association with implantation (Zaninovic et al., 2001; Ahlström et al., 2011), whereas other publications have found no relationship between TE grade and viability (Richter et al., 2001).
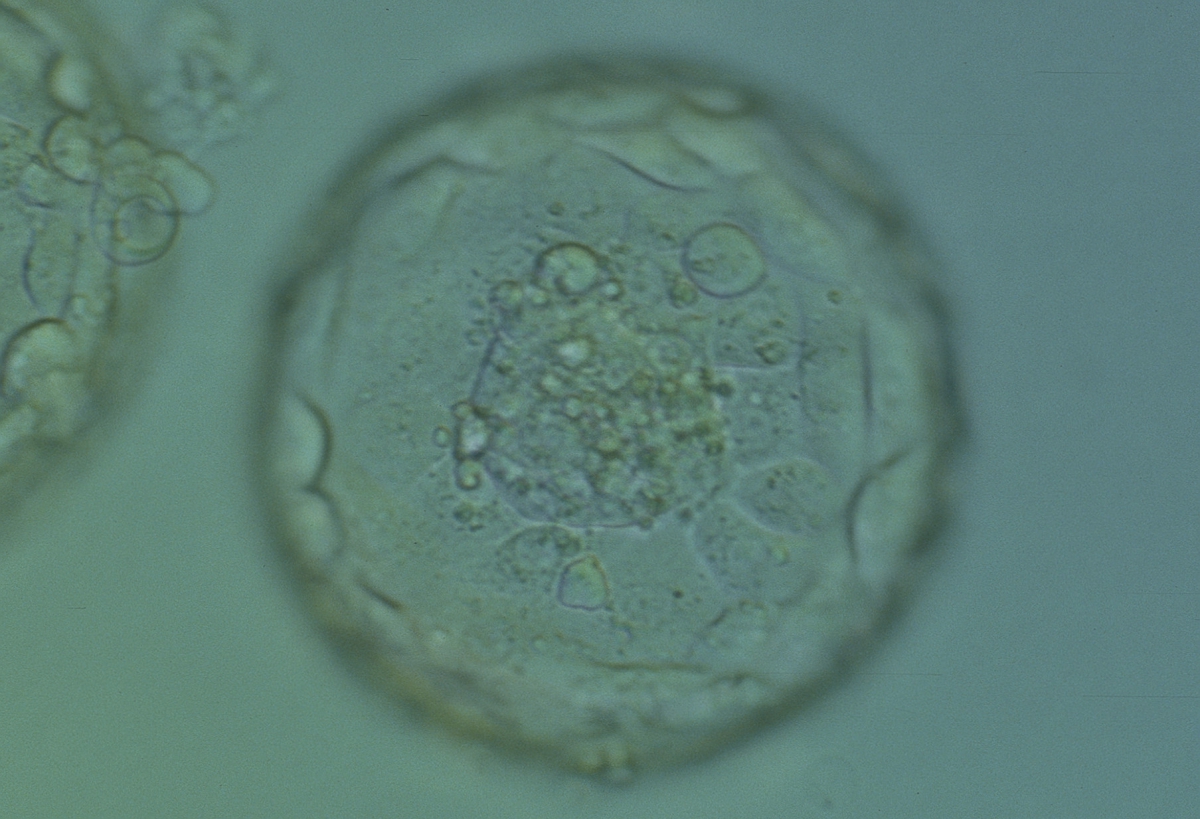
Fong et al. (2001) reported the detailed ultrastructural appearance of TE cells from naturally hatched and enzymatically hatched blastocysts. There was no difference in the ultrastructural appearance of the TE cells following exposure to enzyme: outer tight and adherent junctions, desmosomes, gap junctions, microvilli on the free surfaces, oval-to-tubular mitochondria with well-developed cristae typical of blastocysts, rough endoplasmic reticulum, Golgi complexes, occasional centrioles associated with microtubules, lysosomes, multivesicular bodies and spherical lipid globules present in many cells. Dark granules can be observed in many TE cells at the light microscopic level which are likely to be the lipid globules (Figs 377–379).
Article references:
Ahlström A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting pregnancy and birth after single blastocyst transfer. Hum Reprod 2011;26:3289-3296.
Abstract/FREE Full Text
Aplin JD. The cell biological basis of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol 2000;14:757-764.
CrossRef | Medline | Google Scholar
Fong CY, Bongso A, Sathananthan H, Ho J, Ng SC. Ultrastructural observations of enzymatically treated human blastocysts: zona-free blastocyst transfer and rescue of blastocysts with hatching difficulties. Hum Reprod 2001;16:540-546.
Abstract/FREE Full Text
Richter KS, Harris DC, Daneshmand ST, Shapiro BS. Quantitative grading of a human blastocyst: optimal inner cell mass size and shape. Fertil Steril 2001;76:1157-1167.
CrossRef | Medline | Web of Science | Google Scholar
Sathananthan H, Menezes J, Gunasheela S. Mechanics of human blastocyst hatching in vitro. Reprod BioMed Online 2003;7:228-234.
CrossRef | Medline | Google Scholar
Zaninovic N, Berrios R, Clarke RN, Bodine R, Ye Z, Veeck LL. Blastocyst expansion, inner cell mass (ICM) formation, and trophectoderm (TM) quality: is one more important for implantation? Fertil Steril 2001;76:S8.
Google Scholar